Abstract
Background:
Proteasome inhibitors are currently an integral part of anti-myeloma therapy through all stages of disease. Bortezomib is approved in both the newly diagnosed and relapsed settings. Carfilzomib is currently approved for use in patients with relapsed/refractory multiple myeloma (RRMM), as monotherapy or in combinations with dexamethasone and lenalidomide. Specifically, carfilzomib has been evaluated in patients refractory to or intolerant of bortezomib, with single agent response rate of 23.7% after a median of 5 lines of therapy [Siegel et al. Blood, 2012]. However, to date there is no available data evaluating the use of bortezomib in patients following carfilzomib therapy.
This retrospective study aimed to examine the response rate to proteasome inhibitors used in either of two sequences: bortezomib followed by carfilzomib, or carfilzomib followed by bortezomib. Given the increasing overall survival for patients with myeloma and the rapidly expanding number of available therapies, insight into how to best sequence treatment options is an evolving concern.
Methods:
The response data and treatment summaries of seventeen patients treated with carfilzomib as induction therapy and one hundred fifty-nine patients who received a bortezomib-based regimen as frontline therapy at Weill Cornell Myeloma Center were retrospectively compared. Eligible patients had received therapy with both bortezomib and carfilzomib, in this sequence or in reverse, (B-C or C-B respectively). Data was obtained from participants in a number of trials at the institution for whom the most robust response data was available. Response to treatment was assessed by International Myeloma Working Group (IMWG) criteria [Palumbo et al. JCO, 2014]. Descriptive statistical analysis was then implemented to gauge differences in the two arms.
Results:
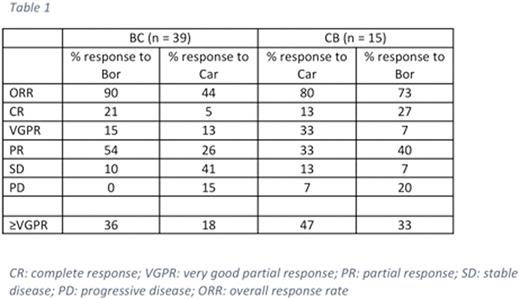
Thirty-nine eligible patients comprised the B-C group and fifteen patients comprised the C-B group. Patients in the B-C group were more extensively pre-treated with a median of 4 lines of therapy before their second proteasome inhibitor (range 2-11) compared to a median of 2 lines in the C-B group (range 2-3). Median cycles of bortezomib and carfilzomib in the B-C group were 8 (range 2-41) and 8 (1-60), respectively. Median cycles of carfilzomib and bortezomib in the C-B group were 5 (range 1- 9) and 5 (1-35), respectively. Responses are shown in Table 1.
Discussion:
Response rates were similar in the two groups, supporting the role of bortezomib-based therapy in patients previously treated with carfilzomib. Bortezomib in the salvage setting tended not only towards higher response rates, but deeper responses as well. It should be noted that patients in the B-C arm of the study were more likely to have undergone more prior lines of therapy than those in the C-B arm, which may have contributed to more resistant disease. We also acknowledge the small cohort for whom data was available. Currently standard of care is to treat myeloma with bortezomib upfront followed by salvage carfilzomib. As data becomes available for use of frontline carfilzomib it is important to know that bortezomib provides a powerful salvage option.
With a rapidly expanding armamentarium of novel agents and increasing time over which to employ them, it will be valuable to learn the optimal sequence of their application to achieve the best and long lasting responses. Our results support the need for further evaluation of proteasome inhibitor sequencing in patients with myeloma.
Rossi:Takeda: Speakers Bureau; Onyx: Research Funding, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Janssen: Speakers Bureau. Perry:Celgene: Speakers Bureau. Pekle:Millenium: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Mark:Millenium: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Onyx: Research Funding, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Niesvizky:Celgene: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy, Research Funding, Speakers Bureau; Onyx: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal