Abstract
Carfilzomib (CFZ) combinations have shown activity in phase 2 and 3 studies in multiple myeloma (MM) patients but also a small but consistent signal of cardiac and renal toxicity. The aim of our study was to analyze potential cardiac and renal toxicity of CFZ combinations in consecutive MM patients.
The analysis included 60 patients treated with different combination of CFZ in a single center (University of Athens, Greece): 48 (80%) had relapsed or refractory (RRMM) and 12 (20%) had newly diagnosed MM (NDMM). All had baseline evaluation of cardiovascular risk factors and echocardiography with a LVEF ≥40%. Regimens included Kd in 31 (52%), KRd in 17 (28%) and KMP in 12 (20%) patients. CFZ dose was 20/27 in 27 (45%) patients, 20/36 in 12 (20%) and 20/56 in 21 (35%). Median age was 72 (range 39-76) years and 65% were males. Median number of prior therapies was 2 (range 0-7). Median baseline eGFR was 88 ml/min (range 16 to> 120 ml/min), 33% had eGFR <60 ml/min and 7% had eGFR <30 ml/min.
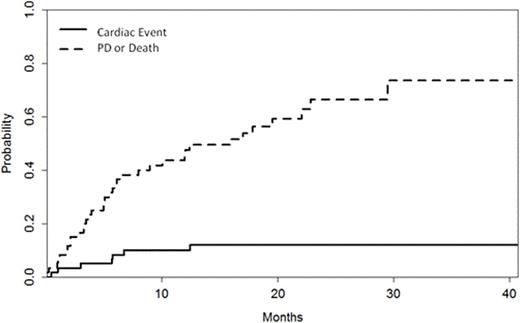
Median duration of CFZ therapy was 10 (range 1-43) cycles. During therapy with CFZ, 7 (12%) patients had a reduction in LVEF ≥20% within a median of 6 months (range 1-13 months) from initiation of CFZ. In 6/7 patients, dyspnea of grade (gr) ≥2 was also present and was associated with a significant increase of NTproBNP (median 2412, range 2219-10162 pg/ml) without increase in troponins. With discontinuation because of disease progression or other unrelated reasons as a competing event, the incidence of LVEF reduction ≥20% was 5% at 3 months, 8% at 6 months, 10% at 12 months and 12% at 15 months. The respective CFZ discontinuation rate unrelated to cardiac toxicity was 17%, 35%, 41% and 49%, respectively (Figure). Among cardiovascular risk factors (age, smoking, hypertension, hypelipidemia, diabetes, renal dysfunction) only peripheral artery disease was associated more often with cardiac events (3/7, 43% vs 4/53,8%, p=0.02). The use of ACE-I, ARBs or CCBs was not associated with cardiac events, however, the use of b-blockers was more common in patients who had LVEF reduction (3/10, 30% vs 4/50, 8%, p=0.048); patients on b-blockers also experienced LVEF reduction earlier [2% vs 20% at 3 months, 6% vs 20% at 6 months and 6% vs 30% at 12 months (p=0.02)], while non-toxicity discontinuation at 12 months was 44% vs 57% (p=0.66). There was no association with prior anthracycline exposure or prior HDT and the dose of CFZ was not associated with cardiac event frequency or timing. Baseline parameters of cardiac function assessed by echocardiography did not show correlation with cardiac events. Further evaluation in all patients who had EF reduction established the diagnosis of coronary artery disease (CAD) in 3/7. In all patients LVEF improved after holding CFZ (<28 days) and 6/7 patients continued therapy with CFZ with dose reduction; only in one patient reinstitution of CFZ was associated with repeated reduction of LVEF.
We also evaluated the effects of CFZ in renal function, excluding renal dysfunction associated with disease progression. Per CTCAE v4.03, 22 (37%) patients had a creatinine increase ≥gr 1 (18% gr1, 17% gr2 and 2% gr3). According to the same criteria for acute kidney injury (AKI), 17 (28%) patients experienced AKI ≥gr 1 (gr1 in 25% and gr3 in 3%) while 21 (35%) had a reduction of their eGFR by ≥25%, which was transient in 13/21 (62%). These events occurred mostly early, within the 1st cycle in 9/21 (43%). Two patients, both in CR, developed TTP, one after 22 and the other after 8 cycles of CFZ. Higher doses of CFZ were associated with higher frequency of eGFR reduction ≥25% (22% vs 33% vs 52% for 20/27, 20/36 and 20/56 doses respectively, p=0.093). Age >65 years was not associated with higher risk of AKI (25% vs 35% for those <65 years, p=0.3). Among patients with baseline eGFR <60 ml/min (n=20), 11 (55%) patients improved their eGFR to >60 ml/min.
In conclusion, cardiac toxicity after CFZ occurred in 12% of patients, but was essentially unpredictable and reversible in all patients; standard cardiovascular risk factors were not predictive of cardiac toxicity. However, in about half of the patients was associated with underlying CAD, indicating that further investigation is needed regarding the effects of CFZ to vascular function and endothelium. Decrease in eGFR and low grade AKI is common but transient, and can be associated with higher doses of CFZ. Importantly, 55% of patients with moderate CKD improved their renal function after treatment with CFZ.
Dimopoulos:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria; Genesis: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Terpos:BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Travel expenses, Research Funding; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Other: Travel expenses, Research Funding; Genesis: Consultancy, Honoraria, Other: Travel expenses, Research Funding; Celgene: Honoraria; Novartis: Honoraria. Kastritis:Genesis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Janssen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal