Abstract
Introduction
Ibrutinib is a first-in-class, once-daily, oral, covalent inhibitor of Bruton's tyrosine kinase (BTK) that as a single agent has significantly improved overall survival in patients (pts) with both treatment naïve and relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). In the phase 3 HELIOS trial, the addition of ibrutinib to bendamustine + rituximab (BR) resulted in an 80% reduction in disease progression or death (HR, 0.203, 95% CI, 0.150-0.276; p < 0.0001) and confirmed for the first time, in a randomized setting, the benefit of ibrutinib-based therapy compared with standard chemoimmunotherapy in previously treated pts (Chanan-Khan, et al. Lancet Oncol. 2016;17(2):200-11). Independent Review Committee assessed progression-free survival (PFS) at 18 months was 79% (95% CI: 73%-83%) in the ibrutinib +BR (I+BR) arm and 24% (95% CI: 18%-31%) in the placebo + BR (P+BR) arm. While the activity of ibrutinib is primarily in B-cells where it inhibits BTK-dependent survival pathways, it may also have significant effects on the immune system by exerting an effect on T-cells (by inhibition of interleukin-2-inducible kinase and related shift from Th2 to Th1-based immunity, which in turn affects Treg and Th17 activity) and via normalization of immune function, thus affecting tumor response. Here, we report changes in circulating T-cell immunophenotypes in a subset of pts treated with either I+BR or P+BR in the HELIOS study.
Methods
HELIOS is a randomized, double-blind, placebo-controlled, phase 3 study. Pts with active CLL/SLL following ≥ 1 prior therapy were randomized 1:1 to receive BR (≤ 6 cycles) with either ibrutinib (420 mg daily; n = 289) or placebo (n = 289). Pts with del17p (> 20% of cells) were excluded. Peripheral blood was collected at the start of the study, at Cycle 1, Day 15 (C1D15) and at the end of treatment/time of progressive disease (EOT/PD). From these samples, peripheral blood mononuclear cells (PBMC) were separated and cryopreserved. PBMC were subjected to flow-cytometric analysis of T-cell subsets including CD4, CD8 and markers indicative of Treg cells (CD25+CD127low) and Th17 cells (CCR4+CCR6+). In addition, expression of T-cell checkpoints ICOS, PD-1 and OX-40 by CD4 and CD8 cells were evaluated as an indicator of cell activation or activation/exhaustion. The findings from the HELIOS study reported here are based on analysis of paired samples (C1D1 and C1D15) of 29 CLL cases from the I+BR arm and 22 cases from the P+BR arm. The Mann-Whitney U test was applied to determine the significance of the differences in each of T-cell subtype between the two arms.
Results
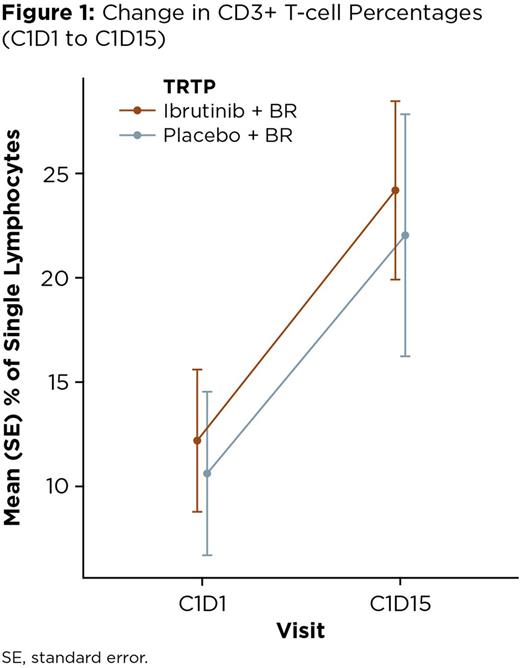
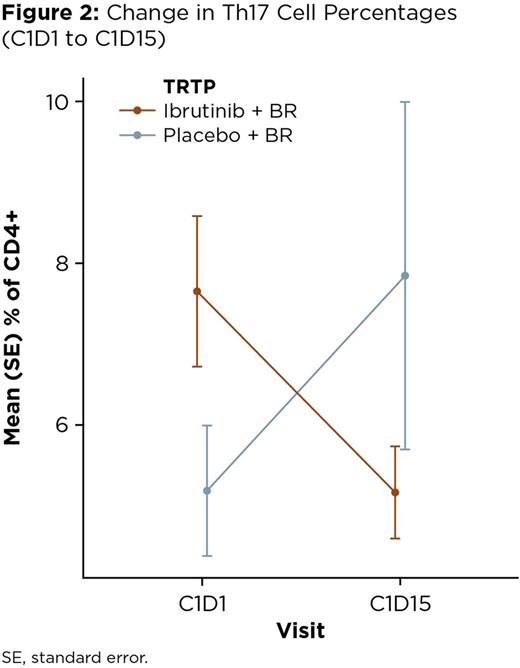
Overall, there was a net increase in the percentage of CD3+ cells with treatment in both the I+BR-treated pts and P+BR-treated pts, which was observed as early as Day 15 (+12.0% and +11.42% of total lymphocytes, respectively, at C1D15 compared with C1D1; Figure 1). The EOT/PD samples showed a continued increase in the percentage of CD3+ cells in the I+BR pts, the majority of whom (11/12) were responders, while the opposite trend was observed in the P+BR patients (8/21 responders). Focusing on the early changes in the analysis of T-cell subsets, a pronounced decrease in the percentage of Th17 CD4+ cells was noted in the I+BR pts (mean: -2.49%) but in the P+BR pts, an increase of this subset (mean: +2.66%) was observed (p = 0.011; Figure 2). When comparing other CD4+ subsets, decreases in Treg cells were seen in both pt groups, with a corresponding increase in the T-cell activation marker ICOS. Less significant changes were observed in the other T-cell markers studied, however, a trend to increased PD-1 was seen in the P+BR pts but not in the I+BR pts. The safety profile in these pts was consistent with previous reports for the study.
Conclusions
These results suggest that the addition of ibrutinib to BR helped restore T-cell proportions in this subset of cases and it is noteworthy that this effect was visible even within 15 days of initiation of therapy. Data from the I+BR pts showed a pronounced decrease in the percentage of Th17 CD4+ cells, which are primarily pro-tumorigenic but may sometimes have anti-tumor effects, and a concomitant decrease in the percentage of Treg cells, which may explain the overall increase in T-cell activation. These findings may also be related to the observed increase in PFS with the addition of ibrutinib to BR reported in the trial.
Damle:Janssen Research & Development: Employment. Schaffer:Janssen Research & Development: Employment. Chaturvedi:Janssen Research & Development: Employment. Phelps:Johnson & Johnson: Employment, Equity Ownership. Aquino:Janssen Research & Development: Employment. Mahler:Janssen Research & Development: Employment. Salman:Janssen Research & Development: Employment, Equity Ownership, Other: Travel, Accommodations, Expenses. Howes:Janssen Research & Development: Employment. Loscertales:Janssen Research & Development: Consultancy; Roche: Consultancy; Gilead: Consultancy; AbbVie: Consultancy. Trneny:Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Research & Development: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Balasubramanian:Janssen Research & Development: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal