Abstract
Chronic Lymphocytic Leukemia (CLL) has a varied clinical course; some patients experience a long survival and others succumb to disease in a short time. Clinical factors correlated with either time to first treatment (TFT) and/or overall survival include Rai stage, IGHV somatic hypermutation status, fluorescence in situ hybridization (FISH) abnormalities, especially del(17p), karyotypic complexity and the presence of a cytogenetic translocation. Previous studies have included patients both at diagnosis and at various times throughout their diseases, and many included limited numbers of patients, precluding extensive analyses of relationships between the prognostic factors and their relative impact on clinical outcome. We sought to identify which factors determined within a short time of diagnosis (i.e., 1 year) were prognostic for TFT in untreated CLL patients.
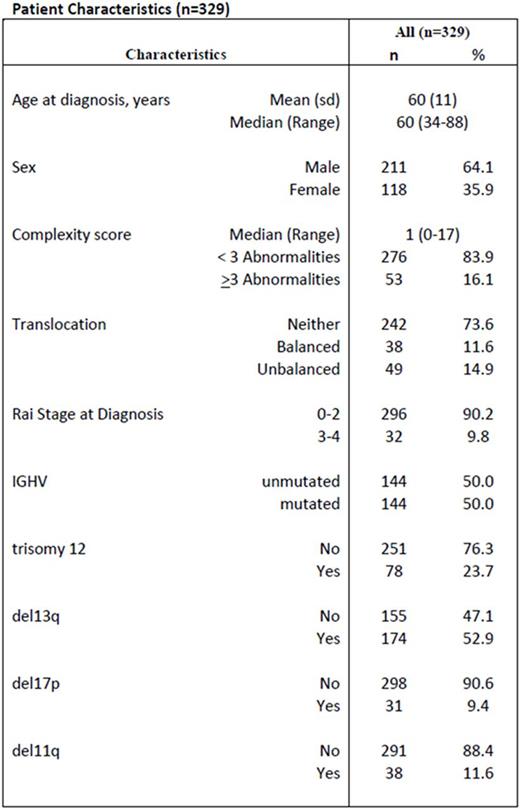
We identified 329 untreated CLL patients who had stimulated karyotypic and FISH analyses within 1 year of diagnosis seen at The Ohio State University (OSU). Patient characteristics and outcome were obtained from patient records. The studies were approved by the OSU IRB and were conducted according to the Declaration of Helsinki. A complex karyotype was defined as ≥ 3 unrelated aberrations by karyotype. Patient characteristics are given in Table 1. Translocations occurred in 87 (26.4%) patients: 38 balanced and 49 unbalanced translocations. Initial statistical analyses showed no large difference in TFT between balanced and unbalanced translations, so they were combined for final analyses. 144 patients (49 with and 95 without a translocation) had unmutated IGHV, and 144 patients (22 with and 122 without a translocation) had mutated IGHV. IGHV data were not available for 41 patients.
TFT was calculated from date of diagnosis to date of first treatment. Untreated patients were censored at last known untreated date. Kaplan-Meier curves estimated TFT probability, and proportional hazard models were used to examine the association between potential risk factors and TFT. Using backward selection, variables with statistical significance when adjusting for all other covariates were included in the final model. To evaluate potential effect modifications, pairwise interactions among all the variables in the final model were examined and retained if statistically significant. Stata 14 (College Station TX) was used, and all tests were two-sided with statistical significance set at p<0.05.
Median follow-up for censored patients was 30 months (range 0.03-102 months). Median TFT for the entire cohort was 47 months (95% confidence interval (CI) 40-63 months). In a univariable model, the following factors were significant: presence of a translocation (hazard risk (HR) 2.69, CI 1.91-3.78, p<0.001), Rai stage III/IV (HR 3.73, CI 2.32-5.99, p<0.001), complexity (HR 2.92, CI 1.98-4.31, p<0.001), unmutated IGHV (HR 3.54, CI 2.42-5.17, p<0.0001), del17p (HR 2.10, CI 1.31-3.37, p=0.002), del11q (HR 2.91,CI 1.92-4.40, p<0.001). In the multivariable model, there was significant effect modification of IGVH status on the relationship between translocation and TFT (p<0.001). In IGHV mutated patients, those with a translocation had over 5 times the risk of starting treatment relative to those without a translocation (HR 5.30, CI 2.76-10.17); however, in IGHV unmutated patients, a translocation did not significantly increase the risk of starting treatment (HR 1.32, CI 0.86-2.03). Independent of IGHV and translocation, Rai Stage (HR 2.07, CI 1.24-3.45, p=0.01) and del11q (HR 1.68, CI 1.09-2.60, p=0.02) were the only variables that remained statistically significant. Notably, once these variables were accounted for in the model, complexity did not provide additional significant prognostic information (p=0.12), perhaps due to its strong association with a translocation (p<0.001). In summary, the presence of a translocation in IGHV mutated patients appeared to negate the improved prognosis associated with mutated IGHV, but the presence of a translocation did not have an effect on TFT in high-risk IGHV unmutated patients (Figure 1).
Time to Treatment for patients with vs without a translocation and with mutated vs unmutaed IGVH
Time to Treatment for patients with vs without a translocation and with mutated vs unmutaed IGVH
Jones:Pharmacyclics, LLC, an AbbVie Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Andritsos:Hairy Cell Leukemia Foundation: Research Funding. Woyach:Morphosys: Research Funding; Acerta: Research Funding; Karyopharm: Research Funding. Awan:Pharmacyclics: Consultancy; Novartis Oncology: Consultancy; Innate Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal