Abstract
Background: In the AZA-001 trial, azacitidine (AZA) altered the natural history of patients with higher-risk MDS (Fenaux et al., Lancet 2009) by significantly improving overall survival compared to conventional care regimens (24.5 months versus 15.0 months). Once approved for drug reimbursement by the provincial health ministry, Cancer Care Ontario (CCO) mandated that all eligible patients be enrolled in a prospective registry to ensure compliance with eligibility criteria and schedules of drug administration. Baseline characteristics were recorded and treatment response were to be submitted every 6 cycles of treatment. Our objectives were to audit this clinical program for results after 6 years and identify the prognostic markers for survival in a homogenous higher-risk MDS/low blast count AML patient population.
Methods: Only higher-risk MDS patients (intermediate-2, high) as defined by the International Prognostic Scoring System (IPSS) and low blast count AML (20-30% blasts) treated with AZA in Ontario, Canada from June 1, 2010 to March 2, 2016 were eligible and included. Our primary outcome was overall survival (OS) from date of first AZA treatment. Our secondary outcomes were overall response rates and the predictors of OS including administration schedules, centre size or type (regional cancer versus community). Univariate and multivariable Cox proportional hazard model were used to determine the predictors of OS. Hazard ratios and generalized R2 (higher the R2, stronger association with OS) were also calculated.
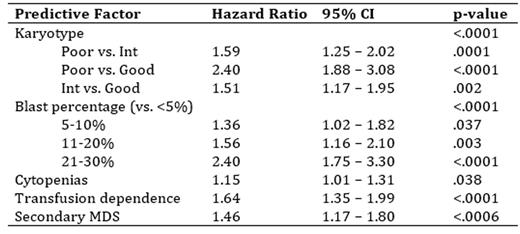
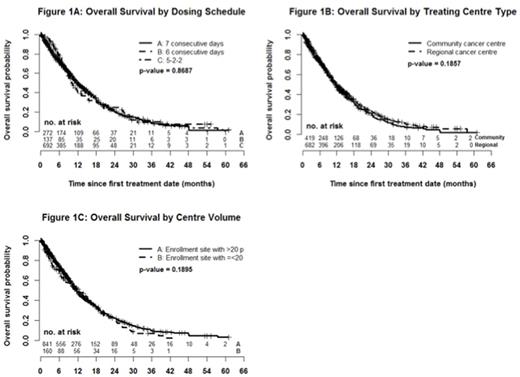
Results: 825 higher-risk MDS and 276 low blast count AML were included (n = 1101 total). Median age was 74 years (range 19 to 99), 65.2% were male and the IPSS scores were intermediate-2 (64.3%) and high-risk (35.7%). Sixty-six percent of patients were transfusion dependent (TD) at time of AZA initiation and 15.5% had received previous chemotherapy. By dosing schedule, 24.7% received AZA for 7 consecutive days (7d), 12.4% for 6 consecutive days (6d) and 62.9% by the 5-2-2 schedule. Overall, the median number of cycles received was 6 (range 1 to 67) and 8 (range 6 to 14) when restricted to the 692 (63%) patients who received at least 4 cycles of treatment. Dose reductions were seen in 33.3% of patients (mean 11.1 mg/m2 in those with reductions) and were more common over time (negative slope 0.178; p < .0001). Of those with repeat bone marrow (n = 293) best response was complete response (CR) in 16.7% and partial response (PR) in 10.6%. Of those without CR/PR/progressive disease on bone marrow (n = 814), 20.4% experienced hematologic improvement including those with marrow CR and marrow stable disease. The actuarial median survival was 11.6 months (95% CI 10.7- 12.4) with a significantly longer OS for MDS compared with low blast count AML (12.4 months vs. 9.6 months; p = .0002) and 16.7 months (95% CI 15.2-18.1) for those receiving at least 4 cycles. There was no difference in OS between the 3 dosing schedules (11.7 months (7d) vs. 10.2 months (6d) vs. 12.0 months (5-2-2); p = .87; figure 1A), regional cancer centre vs. community (11.3 months vs. 11.7 months; p = .19; figure 1B) or by centre volume (11.4 months < 50 patients vs. 11.6 months > 50 patients; p = .38; figure 1C). On univariate analysis, the following were predictive of OS: blast percentage, number of cytopenias, karyotype, IPSS, WHO classification, TD, greater transfusion burden and secondary MDS. The multivariate model with the highest R2 (13.5%) included blast percentage (p < .0001), karyotype (p < .0001), cytopenias (p = .038), TD (p < .0001) and secondary MDS (p = .0006) as summarized in the table below.
Conclusions: In our large real world evaluation of AZA use in higher-risk MDS/low blast count AML, we validated the expected overall response rates to AZA but demonstrated a lower than expected OS compared to the AZA-001 trial. Reassuringly, survival did not differ by dosing schedules, centre volumes or center type (regional cancer vs. community). OS was higher in the 2/3 of patients who received at least 4 cycles of treatment, reinforcing the necessity of sustained administration until therapeutic benefits are realized. This represents the largest real world evaluation of AZA in higher-risk MDS/low blast count AML and additional analyses are underway.
Mozessohn:Celgene: Honoraria. Buckstein:Celgene: Honoraria, Research Funding; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal