Abstract
Patients with Hodgkin lymphoma (HL) who relapse or are refractory to first-line therapy have a poor prognosis and some of these patients require innovative therapeutic approaches. Our group and others have demonstrated the clinical efficacy of anti-CD19 chimeric antigen receptor T cells (CART19) for B cell malignancies. However, B cell antigens such as CD19 are typically absent from Hodgkin Reed-Sternberg (HRS) cells. Targeting the CD30 antigen using CART cells has met with limited clinical success to date. In HL the neoplastic HRS cells often represent ~1% of the tumor mass and the remainder is comprised of a highly immunosuppressive tumor microenvironment (TME). Within the TME, tumor-associated macrophages (TAM) have a key role in promoting tumor growth and inhibiting the anti-tumor immune response, and the presence of TAM in HL is associated with a poor prognosis. HL therefore represents an excellent model of tumor-induced immunosuppression that can potentially represent a barrier to successful CART therapy. The objective of this study was therefore to develop an anti-HL CAR T cell immunotherapy that would also overcome the immunosuppression of the HL microenvironment.
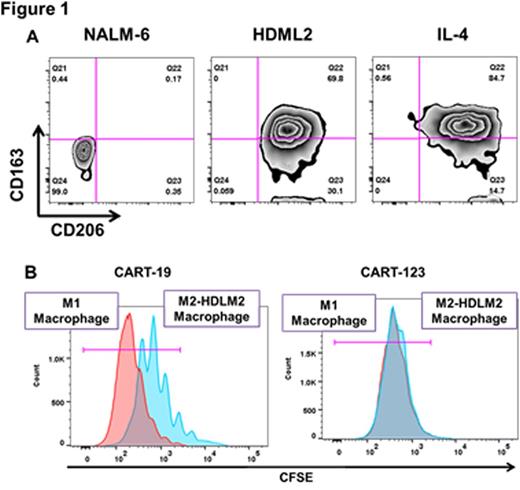
We sought to define a tumor antigen that was co-expressed on HRS and on TAM. Using immunohistochemistry and flow cytometry we confirmed previous reports that CD123 (the IL-3Rα chain) is expressed on HRS in 50-60% of HL patients and in 100% HL cell lines (HDLM-2, KMH2, SUPHD1, and L428). Importantly, CD123 was also seen on several HL-associated immune cells of the TME, including TAM. Exposure of macrophages to HDLM-2 cells polarized them to an M2-like phenotype (Figure 1 A) with expression of CD123. Luminex analysis of HDLM-2/TAM co-culture supernatants revealed high levels of IL-13, and blockade of IL-13 partially reversed the immunosuppressive phenotype. We then developed a model of TAM-mediated CAR T cell inhibition, using immunosuppressive M2-like macrophages derived by exposure to IL-4 or to HDLM-2 cells. These macrophages could suppress “third party” CART19 proliferation in response to B-acute lymphoblastic leukemia. We then used anti-CD123 CART cells (CART123) to target TAM. Importantly, CART123 actively recognized and killed TAM, thereby resisting TAM-mediated inhibition (as opposed to control CART19 cells) (Figure 1 B).
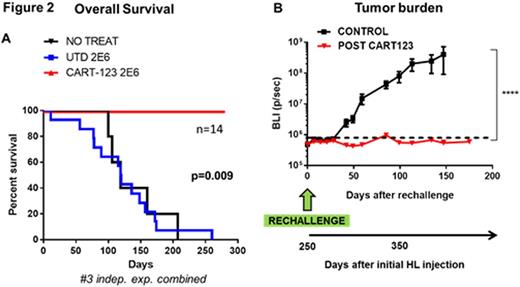
We therefore sought to investigate the utility of anti-CD123 CART for the treatment of HL (initial results for this part presented at ASH 2014). In vitro, CART123 specifically degranulate, proliferate, produce cytokines and kill HL cells. A clinically-relevant xenograft model of HL was generated using luciferase+ HDLM-2 cells in NSG mice. In this model mice showed progressive increase in tumor burden over approximately 6 weeks, reproducing the indolent nature of the human disease. At day ~43 mice were randomized to receive 1.5 million CART123 cells or control T cells. CART123 induced complete and durable eradication of disseminated tumor within 14 days, leading to 100% relapse-free and 100% overall survival at 8 months while mice treated with control T cells had a median survival of 128 days (Figure 2 A). Tumor elimination was associated with extensive CAR T cell expansion as detected by flow cytometry in serial peripheral blood bleedings. In order to prove the formation of an immunological memory, at day 250 CART123 treated mice were re-challenged with the HDLM-2 cell line. The tumor was promptly rejected, associated with a re-expansion of CART123 cells in the peripheral blood (~10 months after T cell injection) leading to an overall survival advantage as compared to controls (Figure 2 B).
In summary, we showed that human CD123-redirected T cells display potent therapeutic activity against disseminated HL. In Hodgkin Lymphoma (HL) CD123 is expressed on malignant Reed-Sternberg cells as well as in M2-polarized tumor associated macrophages (TAMs): importantly CART123 can target simultaneously both the HRS and the TAM without being resistant to immunosuppression. We have previously demonstrated that CART123 can lead to myelosuppression in preclinical models, suggesting that our findings could be translated to treat patients with refractory HL in the context of stem cell transplantation using “short-acting” RNA-CAR123 T cells or depletable lentivirally-transduced CART123.
Ruella:Novartis: Patents & Royalties. Kenderian:Novartis: Patents & Royalties, Research Funding. Wasik:Gilead Sciences: Equity Ownership; Seattle Genetics: Honoraria; Novartis: Research Funding; University of Pennsylvania: Patents & Royalties: NPM-ALK as an omncogene; University of Pennsylvania: Patents & Royalties: CAR T-cells; Gilead Sciences: Research Funding; Pharmacyclics: Research Funding. June:Novartis: Honoraria, Patents & Royalties, Research Funding; Johnson & Johnson: Honoraria; Pfizer: Honoraria; Celldex: Consultancy, Equity Ownership; Immune Design: Consultancy, Equity Ownership; Tmunity Therapeutics: Equity Ownership; Novartis: Honoraria, Patents & Royalties, Research Funding. Gill:Novartis: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal