Abstract
Background: JAK2 inhibition improves splenomegaly and symptoms in patients with myelofibrosis (MF), and the JAK2 inhibitor ruxolitinib has been shown to improve both bone marrow fibrosis and survival in MF patients (Cervantes et al., 2013; Verstovsek et al., 2013). Current prognostic models such as DIPSS plus (Gangat et al., 2011) predict survival in MF based on clinical, laboratory, and cytogenetic information, but predate the era of JAK2 inhibitor use in MF. As such, their value in predicting clinical response or survival during treatment with JAK2 inhibitors remains unknown. We hypothesized that features not considered by DIPSS plus, including bone marrow fibrosis and splenomegaly, have independent effects on therapy response. We therefore conducted a retrospective analysis to create a new model to risk stratify patients with respect to their likelihood of achieving a > 50% reduction in splenic size by palpation with JAK2-inhibitor therapy.
Methods: We studied a cohort of 418 patients with bone marrow biopsy-proven MF who were treated with ruxolitinib or one of four other JAK2 inhibitors. An initial cohort of 203 patients seen at University of Michigan, Stanford University, and Mayo Clinic in Scottsdale, AZ was used identify factors that would predict a clinical response to therapy. Response to therapy was evaluated at an early time point (3-4 mos) in 167 patients and at a late time point (5-12 mos) in 138 patients, with 127 patients overlapping between the two. We constructed a multivariable logistic regression model, with Bayesian variable selection, for predicting the probability of early or late splenic response to JAK2 inhibitor therapy. The model was developed with this initial group of 203 patients. The model was then validated with a cohort of 215 patients from the PERSIST1 trial (data supplied by CTI Biopharma).
Results:
Conclusion: We identified several factors that play a role in predicting whether or not patients with MF are likely to respond to JAK2 inhibitor therapy. The interaction between spleen size and WBC at the time of therapy initiation plays the strongest role in predicting which patients will have an early response to therapy. Additionally, our model suggests that patients who do not have a response to JAK2 inhibitor therapy by about 4 months of treatment are unlikely to develop a later response. Our model is able to discriminate between those likely to benefit after several months of therapy and those for whom a change in therapy should be considered early in their treatment course.
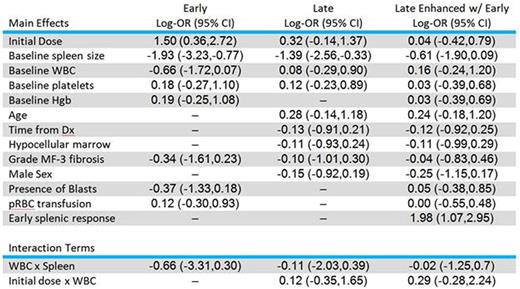
The following characteristics were selected for inclusion in the prognostic model based on the data collected from our 418-patient cohort. Spleen size and white blood cell count at the time of therapy initiation were the strongest predictors of early response to JAK2 inhibitor therapy. Achievement of an early response was used to further enhance the model for the late time point (third column) and was the strongest predictor of late response. Interaction terms looked at the interdependence of variables on splenic response. Log odds ratios are provided with 95% credible intervals in parentheses.
The following characteristics were selected for inclusion in the prognostic model based on the data collected from our 418-patient cohort. Spleen size and white blood cell count at the time of therapy initiation were the strongest predictors of early response to JAK2 inhibitor therapy. Achievement of an early response was used to further enhance the model for the late time point (third column) and was the strongest predictor of late response. Interaction terms looked at the interdependence of variables on splenic response. Log odds ratios are provided with 95% credible intervals in parentheses.
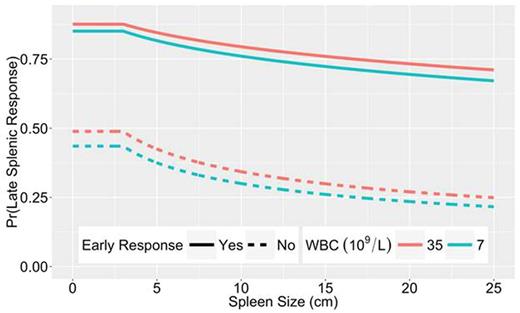
An example of the predicted response for a hypothetical patient. The curves show the interaction of a high initial WBC (red) or a low initial WBC (green) with baseline spleen size (depicted along the X-axis) in predicting whether or not the patient will have a late response to therapy (depicted along the Y-axis). The solid lines show the probability that the patient will respond at the later time point if they had an early response. The dashed lines show the probably of a late response if the patient had not achieved a response by the early time point. Overall, a patient with a higher initial WBC and a larger baseline spleen size is less likely to respond to JAK2 inhibitor therapy. Additionally, the curves clearly separate to show that, for a given baseline WBC and spleen size, a patient who has an early response to therapy is much more likely to have a late response to therapy, whereas a patient who fails to respond early in their treatment course is much less likely to respond with further treatment.
An example of the predicted response for a hypothetical patient. The curves show the interaction of a high initial WBC (red) or a low initial WBC (green) with baseline spleen size (depicted along the X-axis) in predicting whether or not the patient will have a late response to therapy (depicted along the Y-axis). The solid lines show the probability that the patient will respond at the later time point if they had an early response. The dashed lines show the probably of a late response if the patient had not achieved a response by the early time point. Overall, a patient with a higher initial WBC and a larger baseline spleen size is less likely to respond to JAK2 inhibitor therapy. Additionally, the curves clearly separate to show that, for a given baseline WBC and spleen size, a patient who has an early response to therapy is much more likely to have a late response to therapy, whereas a patient who fails to respond early in their treatment course is much less likely to respond with further treatment.
Boonstra:CTI Biopharma: Research Funding. Gowin:Incyte: Membership on an entity's Board of Directors or advisory committees. Mesa:Novartis: Consultancy; Ariad: Consultancy; Promedior: Research Funding; Celgene: Research Funding; Galena: Consultancy; Incyte: Research Funding; CTI Biopharma: Research Funding; Gilead: Research Funding. Talpaz:Ariad: Other: Expense reimbursement, travel accomodation expenses, Research Funding; Novartis: Research Funding; Incyte Corporation: Other: Travel expense reimbursement, Research Funding; Pfizer: Consultancy, Other: travel accomodation expenses, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal