Abstract
Introduction
The optimal management of primary mediastinal B-cell lymphoma (PMBCL) is not fully established. The addition of rituximab (R) to CHOP-like (cyclophosphamide, hydroxydaunorubicin, vincristine, prednisone) induction therapy has improved survival in PMBCL patients. However, these results were obtained in young preselected patients with a favorable prognosis and a reevaluation in unselected patient cohort is warranted.
Methods
In the current study we retrospectively analyzed the therapy response, progression free and overall survival (PFS, OS) of 80 PMBCL patients treated with a CHOP-based regimen with (N=35, standard treatment since 2001) and without rituximab (N=45). Advanced stage disease was present in 20 patients (25%). Low and low-intermediate or higher risk IPI were found in 50 (66%) and 26 (34%) patients, respectively. Seven patients (16%) in the rituximab cohort and 17 patients (49%) in the non-rituximab cohort received a consolidation high-dose (HD) chemotherapy and autologous blood stem cell transplantation (ABSCT). Radiation therapy was performed in 70 patients (88%). 24 (53%) patients who had received a rituximab-containing induction therapy (overall N=45) received rituximab maintenance therapy. A subgroup analysis with regard to International Prognostic Index (IPI) risk category and consolidation HD chemotherapy and ABSCT was performed.
Results
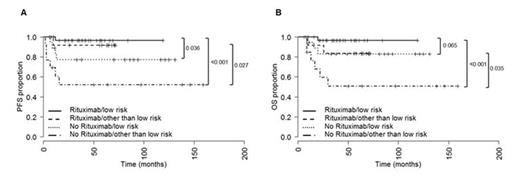
In the cohort treated without rituximab 3 patients (9%) did not respond to therapy, 17 (49%) and 15 (43%) patients reached complete and partial remission (CR, PR). Projected 10-year PFS rate was 67% and projected 10-year OS rate were 72%. In the rituximab cohort no primary induction failures were observed, 15 (34%) and 28 (64%) patients reached CR and PR. Projected 10-year PFS rate was 95% and projected 10-year OS rate was 92%. The differences in PFS and OS between the rituximab and non rituximab cohort were highly significant (PFS P=0.001, HR=0.123, CI95 0.041 - 0.374, OS P=0.023, HR=0.248, CI95 0.079 - 0.778). In the rituximab cohort HD chemotherapy and ABSCT had no significant influence on PFS and OS. Contrary, in not rituximab treated patients a favorable result was observed for patients who received HD chemotherapy and ABSCT with regard to PFS (P=0.043) but not to OS. A subgroup PFS analysis by IPI risk (low versus low-intermediate, high-intermediate, and high) revealed that both low risk and other than low risk PMBCL patients benefit from addition of rituximab to induction chemotherapy (low risk subgroup: P=0.036, HR=7.308, CI95 1.168 - 45.703; other than low risk subgroup: P=0.027, HR=7.442, CI95 1.677 - 33.028, Figure 1A). In addition, the OS probability was significantly higher in the group of low-intermediate, high-intermediate and high IPI risk patients who were treated with rituximab compared to those patients who did not receive rituximab (P=0.035, HR=6.909, CI95 1.564 - 30.511). However, no OS benefit was observed in the low IPI risk subgroup between those patients who received rituximab and those who did not (P=0.065, Figure 1B). No statistically significant differences in PFS (P=0.167) and OS (P=0.585) were observed between patients with and without rituximab maintenance. In multivariate analysis, only addition of rituximab to induction chemotherapy and reaching CR after first line therapy had a beneficial effect on both PFS and OS, whereas IPI, age, upfront HD/ABSCT and rituximab maintenance had no impact on survival.
Conclusion
Our data demonstrate a survival benefit in unselected PMBCL patients treated with CHOP-like induction regimen and additional rituximab compared to CHOP-like treatment without rituximab independently of the IPI risk score. Moreover, if rituximab is added to induction therapy HD chemotherapy and ABSCT as part of the first line therapy do not improve PFS or OS. The role of rituximab maintenance therapy should be further evaluated in larger patient cohorts.
Progression-free survival (PFS, A) and overall survival (OS, B) of low and other than low (low-intermediate, high-intermediate, and high) risk patients by IPI treated either with (N=32 and N=13) or without (N=18 and N=13) rituximab. IPI was not available in 4 patients in the no rituximab treatment group. These patients were excluded from the survival analyses. Median PFS and OS not reached.
Progression-free survival (PFS, A) and overall survival (OS, B) of low and other than low (low-intermediate, high-intermediate, and high) risk patients by IPI treated either with (N=32 and N=13) or without (N=18 and N=13) rituximab. IPI was not available in 4 patients in the no rituximab treatment group. These patients were excluded from the survival analyses. Median PFS and OS not reached.
Witzens-Harig:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal