Abstract
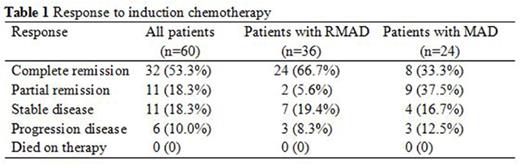
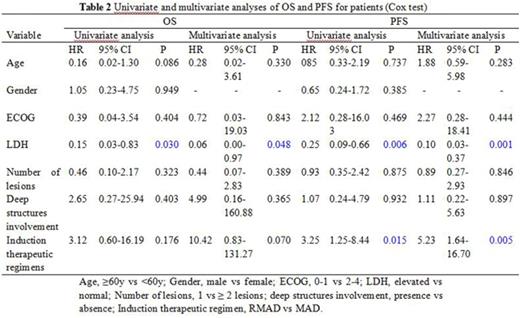
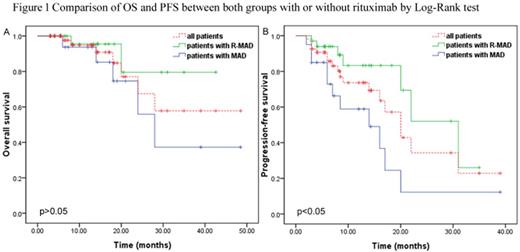
High-dose methotrexate (HD-MTX) based chemotherapy is the standard treatment for patients with newly diagnosed primary central nervous system lymphoma (PCNSL). The role of rituximab is controversial because as a large protein limits its penetration to blood brain barrier (BBB). We aimed to investigate the efficacy and tolerability of adding rituximab to methotrexate-cytarabine-dexamethasone combination therapy (RMAD regimen). We retrospectively analyzed 60 immunocompetent patients with newly diagnosed PCNSL from Beijing Tiantan Hospital, Capital Medical University. Twenty-four patients received 3-6 courses of methotrexate 3.5 g/m2 on day 1, cytarabine 0.5-1 g/m2 on day 2 and dexamethasone 5-10 mg on days 1, 2 and 3. Thirty-six patients received the same combination plus rituximab 375 mg/m2 on day 0. All patients repeated the treatment every 3 weeks. Patients who obtained a complete remission (CR) after induction chemotherapy received consolidation chemotherapy, and those with partial remission (PR), stable disease (SD) or progression disease (PD) received rescue whole brain radiotherapy (WBRT). After induction chemotherapy, patients treated with rituximab plus methotrexate-cytarabine-dexamethasone had a CR rate of 66.7%, compared with 33.3% of those treated with methotrexate-cytarabine-dexamethasone alone (p=.011). Seven anaphylaxis and 1 interstitial pneumonitis occured in patients with rituximab. The most common grade 1-3 adverse events were similar in the two groups including neutropenia, anaemia, thrombocytopenia, aminotransferases elevated and gastrointestinal reaction. None of the patients in the two groups experienced any grade 4 toxicity. On multivariate analysis, rituximab was associated with longer progression-free survival (PFS, hazard ratio: 5.23, 95% CI: 1.64-16.70, p=.005). Elevated serum lactate dehydrogenase (LDH) was associated with shorter OS (hazard ratio: 0.06, 95% CI: 0.00-0.97, p=.048) and PFS (hazard ratio: 0.10, 95% CI: 0.03-0.37, p=.001). HD-MTX based chemotherapy with rituximab had a higher rate of CR, and without serious toxicity. PFS but not OS benefited from addition of rituximab. LDH as an independent prognostic factor which predicted poor outcome.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal