Abstract
Introduction: Severe immune dysregulation as seen in human immunodeficiency virus infection, immunosuppression after solid organ transplant, and autoimmune (AI) disease is known to act as a major risk factor for non-Hodgkin lymphoma (NHL). Recently, the InterLymph Subtypes Project pooled cases and controls to provide well-powered comparisons of risk factors for specific NHL subtypes, such as diffuse large B cell lymphoma (DLBCL), and showed that B cell-activating AI diseases in general (odds ratio 2.4; 95% confidence interval 1.8-3.1) and systemic lupus erythematosus (SLE) and Sjögren's syndrome (SS) in particular were strongly associated with increased DLBCL risk after controlling for all other risk factors (Cerhan J Natl Cancer Inst Monogr. 2014). However, little is known about the demographics or clinical outcomes of DLBCL that arises in the setting of AI disease. We examined the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database to determine the frequency of common B cell AI diseases among older patients with DLBCL and characterize the patterns of presentation, treatment, and survival for DLBCL patients with concomitant AI disease.
Methods: We used the SEER database for patients diagnosed 2002-2009 linked to their Medicare claims data through 2011 to characterize presentation, treatment, and survival patterns in patients with DLBCL, including those with rheumatoid arthritis (RA), SLE, SS, and other B cell-mediated autoimmune diseases as defined by InterLymph criteria (autoimmune hemolytic anemia, Hashimoto's thyroiditis/hypothyroidism, myasthenia gravis; pernicious anemia; Wang Am J Epidemiol. 2015). Patient age, sex, race/ethnicity, region of residence, marital status, year of diagnosis, cause of death, census tract-level characteristics (education, poverty, and metropolitan/urban/rural status), stage, B symptoms, and nodal or extranodal primary site of involvement were identified from the SEER data. Assessments of poor performance status, anemia, the Charlson comorbidity index, survival, and treatment strategies were identified using Medicare inpatient, outpatient and physician claims. Treatments were categorized as: rituximab (R), cyclophosphamide and vincristine (CVP), R-CVP; cyclophosphamide, hydroxydaunorubicin, and vincristine (CHOP), and R-CHOP. Patients who did not receive any DLBCL-directed treatment within 6 months of diagnosis were categorized separately. We examined the baseline clinical characteristics for patients with DLBCL and RA, SLE, SS, or any B cell-mediated autoimmune disease, plotted overall survival and lymphoma-related survival for these groups and compared median survival times.
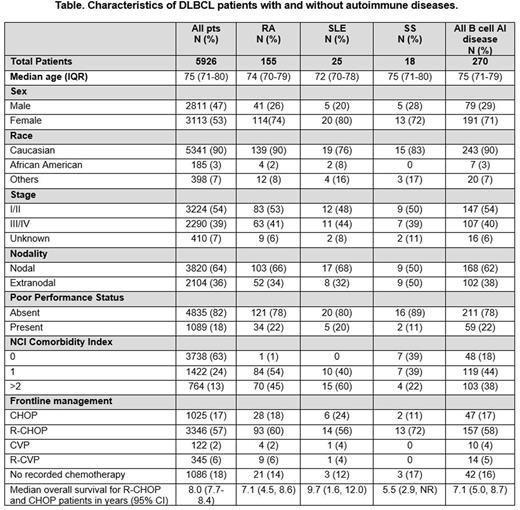
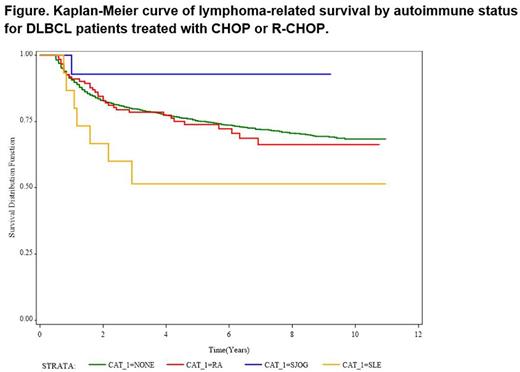
Results: Patient characteristics are summarized in the Table. With the exception that patients with DLBCL and AI disease were more commonly female[KJL1] [CF2] , patients with DLBCL and RA, SLE, SS, or other B cell AI diseases have similar baseline presenting features as other DLBCL patients and received similar first line treatments. There was a trend towards decreased lymphoma-related survival in patients with SLE and DLBCL compared to all other groups, but this difference was not statistically significant in this cohort (Figure) [KJL3] [KJL4] .
Conclusions: In this retrospective claims-based cohort of older patients with DLBCL, concomitant AI disease was uncommon and was more likely to occur in female DLBCL patients, which likely reflects the higher incidence of AI disease in women. The possibility of lower lymphoma-related survival for these patients should be explored in future studies.
Flowers:Millenium/Takeda: Research Funding; NIH: Research Funding; TG Therapeutics: Research Funding; Pharmacyclics, LLC, an AbbVie Company: Research Funding; Mayo Clinic: Research Funding; Infinity: Research Funding; ECOG: Research Funding; Acerta: Research Funding; AbbVie: Research Funding; Roche: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Gilead: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal