Abstract
Background: Due to ~50% risk of relapse with standard therapy (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone, R-CHOP), an increasing number of patients with high risk diffuse large B-cell lymphomas (DLBCL) are being treated with dose-adjusted (DA) EPOCH-R (rituximab, etoposide, doxorubicin, vincristine, prednisone, cyclophosphamide). DA-EPOCH-R contains a 96-hour continuous infusion can be delivered either in the inpatient or outpatient setting, via use of ambulatory infusion pumps. Potential advantages of outpatient therapy include reduced inpatient burden for routine chemotherapy, less exposure to resistant bacterial infections, increased patient satisfaction, and reduced cost. The ability to administer outpatient DA-EPOCH-R is dependent on the ability of the healthcare facility to administer the regimen safely while maintaining dose adjustments and schedule. We hypothesize that patients who receive DA-EPOCH-R as an outpatient have similar outcomes and toxicity rates as patients who receive the regimen as an inpatient. We further hypothesize that there is a significant cost benefit for patients to receive DA-EPOCH-R as an outpatient.
Methods: This was a retrospective database analysis of newly diagnosed consecutive DLBCL patients ≥ 18 years of age who received DA-EPOCH-R chemotherapy at MD Anderson Cancer Center between 2010 and 2014. Patients with double hit lymphoma defined as having a MYC and BCL2 or BCL6 translocation were excluded due to their aggressive nature. We descriptively analyzed demographic variables in this population including, age, gender, international prognostic index (IPI)) and outcome (overall response rates (ORR), complete response (CR), progression free survival (PFS), overall survival (OS), and hospital admission for neutropenic fever events). Additionally, we evaluated the number of outpatient cycles received in relation to survival outcomes and neutropenic fever events. Statistical analysis was done using Fisher's exact test or Chi-square test to evaluate the association between two categorical variables and Wilcoxon rank sum test was used to evaluate the difference in a continuous variable between patient groups. Kaplan-Meier method was used for time-to-event analysis including overall survival and progression free survival. The Log-rank test was used to evaluate the difference in time-to-event endpoints between patient groups.
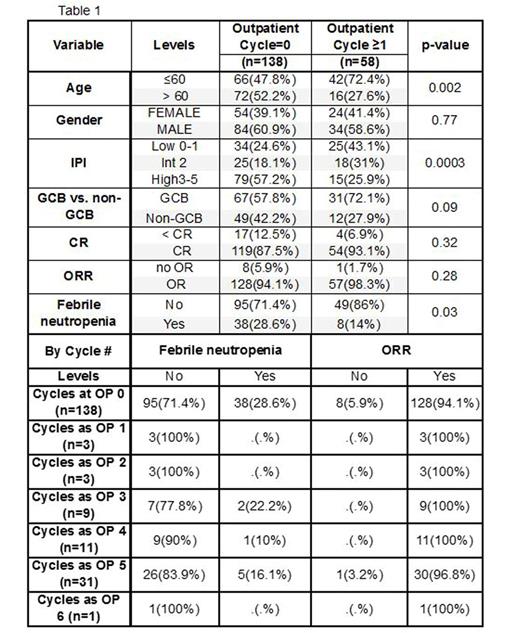
Results: A total of 196 patients had data available for analysis, with 138 patients (70.4%) receiving all cycles as an inpatient, while 58 patients (29.6%) received at least 1 outpatient cycle of DA-EPOCH-R (Table 1). Compared with patients who received no outpatient cycle, the patients who received any outpatient therapy were younger, had a lower proportion of high IPI, and experienced fewer episodes of febrile neutropenia. The median OS and PFS for the entire population has not been reached, with a median follow-up time for the censored observations of 2.78 years (range: 0.24 - 8.64 years). The difference in OS between the patients who had any outpatient therapy and no outpatient therapy was not significant by the log-rank test (p-value=0.11). The difference in PFS between the patients who had any outpatient therapy and no outpatient therapy was marginally significant for OS by the log-rank test (p-value=0.07). Our cost analysis for 6 cycles of inpatient DA-EPOCH-R is estimated to be ~$88K, or $14.6K/cycle. The cost savings incurred for chemotherapy only expenses for each outpatient cycle is at least $3.3K/cycle or $19.8K for a total of 6 cycles.
Conclusion: DA-EPOCH-R is a highly effective regimen for treating aggressive DLBCL which can be administered in an outpatient setting safely, efficaciously, and in a cost-effective manner without any apparent effect on outcome or rate of admission for neutropenic fever. There can be savings of about of nearly $20K per patient for a 6-cycle course of therapy. In our series, patients who received outpatient therapy were younger and may have had greater social support, which could potentially confound results. This retrospective analysis supports the use of outpatient DA-EPOCH-R, but additional studies are warranted to evaluate which patients may benefit most.
Oki:Novartis: Research Funding. Nastoupil:Janssen: Other: Travel, Accommodations, Expenses, Research Funding; TG Therapeutics: Research Funding; Celgene: Honoraria; AbbVie: Research Funding. Fowler:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Research Funding; Janssen: Consultancy, Research Funding. Wang:Asana BioSciences: Research Funding; Acerta: Consultancy, Research Funding; Dava Oncology: Honoraria; BeiGene: Research Funding; Kite Pharma: Research Funding; Juno Therapeutics: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Asana biosciences, Beigene, Celgene, Juno, Kite, Onyx, Pharmacyclics: Research Funding. Fayad:Seattle Genetics: Consultancy, Research Funding. Westin:Celgene: Membership on an entity's Board of Directors or advisory committees; Chugai: Membership on an entity's Board of Directors or advisory committees; Spectrum: Membership on an entity's Board of Directors or advisory committees; ProNAi: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal