Abstract
Background: There are no prospective studies that define best therapy for DHL (presence of c-MYC and BCL-2 translocations) or DEL (co-expression of c-MYC ³40% and BCL-2 ³50% by IHC). Retrospective data for both entities show suboptimal outcomes with R-CHOP and perhaps improved outcomes with intensified regimens such as DA-EPOCH-R. We conducted a phase I prospective multicenter study adding escalating doses of LEN to DA-EPOCH-R in pts with DHL or DEL. Herein, we report the final results of the dose-finding portion of this ongoing clinical trial.
Patients and Methods: Eligible pts had DHL or DEL as defined above. They were allowed to receive radiotherapy for neurologic compromise or one cycle of R-CHOP prior to enrollment at the investigator's discretion. Pts had measurable disease, ECOG PS 0-2, and adequate liver, kidney, and marrow function and no known HIV or CNS involvement. Either aspirin or warfarin prophylaxis was required. Primary objective was to determine the maximum-tolerated dose (MTD) of LEN when added to DA-EPOCH-R. We utilized a standard (3+3 design) where LEN was started at 10mg (days 1-14, Q21-days) with each cycle of DA-EPOCH-R, and escalated to a maximum of 25 mg unless a MTD was reached at an earlier dose. Dose-limiting toxicities (DLTs) were assessed during cycle 1; DA-EPOCH-R administration and dose modifications were conducted as per usual protocol. Cycles were repeated every 3-weeks for a maximum of 6 cycles and were followed by an end of therapy PET scan. CNS prophylaxis was strongly encouraged with 12.5 mg IT-methotrexate for 4 doses during induction. Patients attaining PET negativity after induction were continued on maintenance LEN 10 mg (days 1-14 Q21 days) for 12 cycles. During the LEN dose escalation portion of the study, a hematologic toxicity did not count as a DLT to allow DA-EPOCH-R dose adjustments.
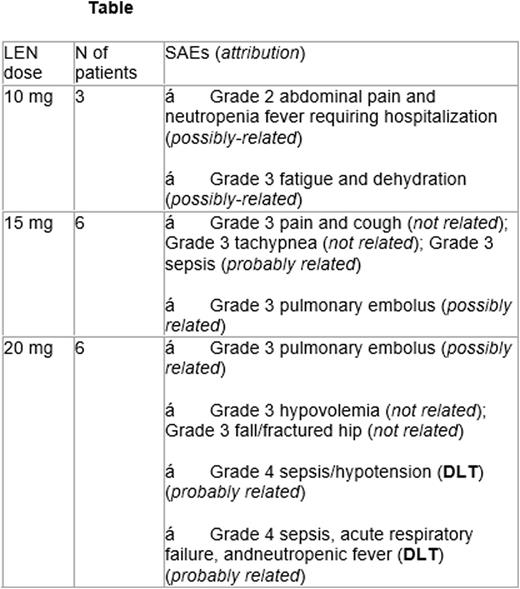
Results: 15 pts (6: DHL and 9: DEL; 13 DLBCL and 2 BCL-U) were enrolled; median age was 62 years (range: 26-83). There were 5 males and 10 females (11 whites, 3 African Americans, and 1 Asian pts). Two pts had ECOG PS 2 while all others were 0-1. All pts (100%) had stage III/IV disease, 13 pts (86%) had high-risk IPI score, and median LDH was 673 (range: 193-1,835). All ptsare assessable for toxicity. Serious adverse events (SAEs) per dose levels are summarized in the Table. DA-EPOCH-R dose escalation was feasible in 10 pts (67%), one pt (6.7%) maintained the same dose throughout, and another (6.7%) required dose de-escalation. Two DLTs were observed (grade 4 sepsis and hypotension for both) at dose-level 20 mg of LEN leading to 15 mg being the MTD and RP2D. Most common grade 1 and/or 2 non-hematologic toxicities were fatigue (70%), constipation (47%), alopecia (53%), nausea (47%), peripheral sensory neuropathy (40%), diarrhea (33%), and hypokalemia (33%). The only grade 3 and/or 4 non-hematologic toxicity occurring in ³2 pts (13%) was hypokalemia. All others occurred in 1 pt (6%) and were: constipation, fever, hyperglycemia, hypertension, mucositis, and hypoalbumenemia. One pt (6%) developed treatment-related myelodysplasia, (T-MDS), 18 months after treatment initiation. Twelve patients are evaluable for response at time of analysis, (1 patient lost to follow-up, and 2 with ongoing therapy). Best responses at completion of induction based on PET were 6 complete responses (CR: 50%), 3 partial responses (PR: 25%) and 1 pt with progressive disease (8%). All CR pts received LEN maintenance but only 1 completed 12 cycles to date (1 came off due to cytopenias found later to have T-MDS, 1 due to AE, 1 due to finding of unrelated colon cancer, 2 on active maintenance). With median follow up 10.7 months (range: 1.3-18.6 months), 14 pts remain alive and one pt has died for an OS of 93%.
Conclusions: LEN can safely be added to DA-EPOCH-R in DHL and DEL patients at a dose of 15 mg (days 1-14 Q21 days). Chemotherapy dose escalation was not compromised and preliminary safety/efficacy data appear promising. A phase II study in this pt population with LEN+DA-EPOCH-R is underway.
Nabhan:Celgene, Genentech, Seattle Genetics, Astellas: Research Funding; Celgene, Genentech, Abbvie, Infinity, Cardinal Health: Consultancy. Karmali:Celgene: Speakers Bureau. Fishkin:Biogen: Other: Owns Stocks. Smith:Genentech: Consultancy, Other: on a DSMB for two trials ; Juno: Consultancy; TGTX: Consultancy; AbbVie: Consultancy; Gilead: Consultancy; Amgen: Other: Educational lecture to sales force; Portola: Consultancy; Celgene: Consultancy; Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal