Abstract
Introduction: Adult T-cell Leukemia-Lymphoma (ATL) is a rare malignancy of T-cells infected with HTLV-1 and has the worst prognosis of the T-cell lymphomas. CC chemokine receptor 4 (CCR4) is overexpressed on ATL cells (>90% of ATL patients [pts]). Mogamulizumab (Moga) is a monoclonal antibody directed against CCR4 that has been approved in Japan for the treatment of CCR4+ ATL. There is no standard or effective treatment for relapsed/refractory (R/R) ATL pts outside of Japan.
Methods: Pts from the USA, EU, and Latin America with R/R ATL (acute, lymphoma, and chronic subtypes) were randomized 2:1 to treatment with Moga, 1.0 mg/kg, given weekly for the first 4-week cycle and then biweekly, or to 1 of 3 investigator choice (IC) regimens (gemcitabine/oxaliplatin, DHAP, or pralatrexate). Pts randomized to the IC arm were permitted to cross over to Moga upon progression. The primary endpoint was confirmed objective response rate (ORR), defined as a response that is maintained for approximately 8 weeks, in those randomized to the Moga arm (based on modified Tsukasaki criteria). ORR was assessed by the treating investigator (IA) and in blinded fashion by independent review (IR). Key secondary endpoints were ORR for IC and after cross over to Moga, duration of response (DoR), progression-free survival (PFS), and overall survival (OS).
Results: 71 pts were randomized (47 to Moga, 24 to IC). Tissue /blood from 65/71 pts (91.5%) expressed CCR4. Although randomized, there were baseline imbalances in known ATL prognostic factors. Pts with adverse prognostic features were more often randomized to Moga: these features included older age, poor ECOG performance status of 2, bone marrow involvement, and refractory to (vs having relapsed from) their most recent ATL therapy. Based on data through 31 March 2016, ORR (all responses, confirmed and unconfirmed) by IA was 34.0% (16/47) in the Moga group and 0% (0/24) in the IC group; these rates were largely concordant with ORR findings by IR of 27.7% (13/47) for the Moga group and 8.3% (2/24) for the IC group. Confirmed ORR for Moga was 14.9%; there were no confirmed responses in the IC group. 18 IC pts crossed over to Moga and 3 responded, including one confirmed response. The median (m) DoR for Moga was 5.65 months [mo] (95% CI 3.63, not reached). Two pts had responses lasting >9 mo. The 16 responders in the Moga arm (as assessed by IA) were evenly distributed over ATL subtypes: chronic 5/16 (31%), acute 5/16 (31%), and lymphoma 6/16 (38%). Of the 65 pts with any exposure to Moga (randomized plus crossover), response according to subtype was: chronic 5/10 (50%), acute 7/31 (23%), and lymphoma 7/24 (29%).
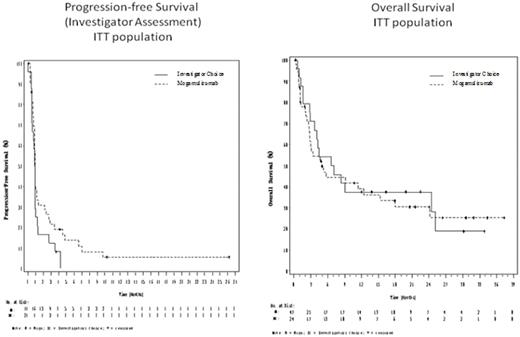
While the study was not powered to demonstrate a difference between the treatment arms, the Kaplan-Meier plots of PFS and OS for the ITT population are presented below. The PFS curves deviate modestly in favor of the Moga group. The mPFS for Moga was 0.93 mo (min-max: 0.03-26.20 mo) with 21.5% of subjects progression free at 3 mo, while the mPFS for IC was 0.88 mo (0-4.23 mo) with 12.5 % of subjects progression free at 3 mo.
The OS curves overlap. The mOS for the Moga arm was 4.90 mo (0.27-37.33 mo) with 38.9% alive at 12 mo, and the mOS for the IC arm was 6.87 mo ( 0.57-33.87 mo) with 37.5% alive at 12 mo. The OS curves may have been affected by the imbalance in prognostic factors and are confounded by the crossover of 75% of IC pts to Moga.
Treatment emergent adverse events (TEAEs) occurring more often in the Moga group than the IC group were infections (51.1% vs 20.8%); respiratory disorders (48.9% vs 29.2%); infusion related reactions (46.8% vs 0%); and skin disorders (42.6% vs 8.3%). TEAEs ≥Grade 3 were 29/47 (61.7%) for Moga and 13/24 (54.2%) for IC. With the exception of known Moga-related events (infusion related reactions and drug eruptions), TEAE rates are similar when adjusted for exposure time to Moga or IC; for example, the AE rate per pt-mo of exposure for infections is 0.296 for the Moga group vs. 0.234 for the IC group.
Conclusions: In the largest randomized clinical trial of pts with R/R ATL thus far conducted, commonly used cytotoxic regimens provided limited to no therapeutic benefit whereas treatment with Moga resulted in objective responses, a trend for favorable PFS, no clear survival advantage or disadvantage, and adverse events that are predictable, thereby supporting Moga's therapeutic potential in this setting.
Phillips:Takeda: Speakers Bureau; Kyowa Kirin: Research Funding; Celgene: Speakers Bureau. Hermine:AB science: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding, Speakers Bureau; Celgene: Research Funding; Novartis: Research Funding; Alexion: Research Funding. Lill:Kite: Research Funding; California Cord Blood Services: Consultancy; Sanofi: Speakers Bureau. Feldman:Seattle Genetics: Consultancy, Research Funding, Speakers Bureau; Abbvie/Pharmacyclics/Janssen: Speakers Bureau; Celgene: Consultancy, Speakers Bureau. Sawas:Gilead Sciences: Speakers Bureau; Seattle Genetics: Honoraria. Cook:Kyowa Kirin Pharmaceutical Development, Inc.: Consultancy. Kurman:Kyowa Kirin Pharmaceutical Development, Inc: Consultancy. George:Kyowa Kirin Pharmaceutical Development: Employment. Dwyer:Kyowa Kirin Pharmaceutical Development, Inc.: Employment. Leoni:Kyowa Kirin Pharmaceutical Development, Inc.: Employment. Gonsky:Bioclinica: Other: Compensation for independent review of trial data. Horwitz:Infinity Pharmaceuticals: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Spectrum Pharmaceuticals: Consultancy, Honoraria, Research Funding; Kyowa Kirin Pharmaceutical: Research Funding; Millennium Pharmaceuticals: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Huya Bioscience International: Consultancy, Honoraria; Forty Seven, Inc.: Consultancy, Honoraria; ADC Therapeutics: Other: Travel.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal