Abstract
Overall survival (OS) remains the gold standard proof of a clinical benefit, and differences in OS can only be determined in randomized Phase 3 clinical studies. However, in rare diseases, the conduct of randomized studies is very challenging, takes protracted periods of time to conduct, can be very expensive while not offering the promise of significant commercial return, and could become clinically irrelevant as other new agents and combinations emerge over the extended time frame of the study. Many drugs approved in this setting are often approved on surrogate end-points like progression free survival (PFS) or complete response (CR) rates in single arm Phase 2 studies. A middle-ground approach could involve using case-matched control analyses (CMCA), which are statistically more robust that single arm Phase 2 studies, and can provide meaningful insights into the likelihood of success in the randomized setting. CMCA require access to large well annotated datasets with a representative patient population, and can be used to benchmark a new drug in a defined patient population. Unfortunately, these types of analyses have not been routinely used in oncology, certainly not in patients with PTCL. We established an integrated international database of patients with R/R PTCL in order to better clarify the clinical benefit and survival advantage of pralatrexate in patients with R/R PTCL, using the original raw data from the PROPEL study.
Data Source. The control population is derived from a collection of four historical databases of patients with R/R PTCL, including: Memorial Sloan-Kettering Cancer Center (MSKCC), University of Nebraska Medical Center (UNMC), Groupe d'Etude des Lymphomes de l'Adulte (GELA), and Samsung Medical Center (SMC). These databases were all annotated with the criteria used to define the PROPEL study population. The cases are from the PROPEL study, an international, multicenter single arm phase II study or pralatrexate in patient with R/R PTCL. The analysis was conducted by an independent statistician in collaboration with investigators from Columbia University.
Method for Matching. The propensity score match was used to match cases and controls. The following characteristics are used in the match process: histology, number of previous treatments received, age at diagnosis (with 20 years interval), and sex. With 1:1 ratio match, the process identified 83 cases and 83 controls. The analysis population includes 68.7% female from cases and 66.3% from controls. For cases and controls, the age distributions are similar: mean age is 57.2 with the range (21 - 83) for cases and 56.7 with range (24 - 89). About 60% of patients received less than three previous treatments in both cases and controls ( 58% vs 59%). More than half of the patients in both cases and controls had histology PTCL-unspecified. Other histology above 10% are AILT and ALCL, primary systemic type.
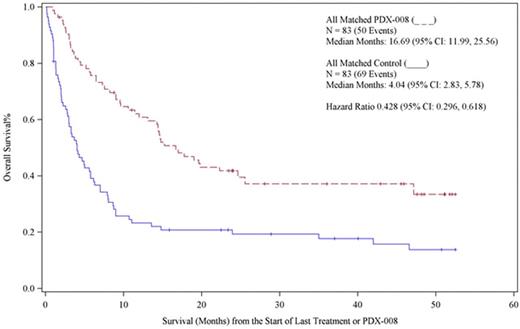
Results. In total, 83 patients out of 109 treated on the PROPEL study were successfully matched with the control population. The cases were matched 1 : 1. Overall survival was plotted for each of the two study populations. The survival curves for the control population were found to be nearly identical to that reported for this population from other datasets, including the ones reported from the International T-Cell Lymphoma Project, the British Columbia experience and the T-Cell Project. The overall survival was 4.04 months (95% CI 2.83, 5.78), which is consistent with historical controls describing the natural history of R/R PTCL in this population. The PROPEL study, which reported an overall response rate of 29% in patients with multiply relapsed PTCL, reported on one of the most diverse and heavily treated populations of R/R PTCL. In PROPEL, the median number of prior therapies was three, with 19% of patient receiving more than 5 lines of prior therapy. The median OS in for the pralatrexate treated cohort in this analysis was 16.6 months (95% CI: 11.99-25.56). There was a highly significant difference in the OS between these two populations, with a hazard ratio of 0.426 (95% CI: 0.296-0/61). The OS curves are shown below. This CMCA demonstrates a highly significant difference in OS in favor of patients treated with pralatrexate. This approach can be used to better understand how new drugs in orphan diseases perform in heterogeneous patient populations. Additional statistical and sensitivity analyses will be reported.
O'Connor:Seattle Genetics: Research Funding; Mundipharma: Membership on an entity's Board of Directors or advisory committees; Spectrum: Research Funding; Seattle Genetics: Research Funding; Spectrum: Research Funding; Mundipharma: Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Research Funding; TG Therapeutics: Research Funding; Bristol Myers Squibb: Research Funding; Bristol Myers Squibb: Research Funding; Celgene: Research Funding; Celgene: Research Funding. Volinn:LLXSolutions,LLC: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal