Abstract
Background: mTOR inhibitors, including temsirolimus (TEM), have demonstrated activity in patients (pts) with relapsed/refractory (rel/ref) lymphomas (J Clin Oncol 2010;28(31):4740). Lenalidomide (LEN) is an immunomodulatory agent with activity in a number of lymphoma subtypes with potential additive or synergistic effects. We previously reported phase I results of TEM/LEN in patients with relapsed/refractory lymphomas and showed good tolerability and evidence of clinical activity (J Clin Oncol 2012;30 suppl; abstract 8075). Recommended phase II doses of TEM 25 mg weekly and LEN 20 mg daily (D1-21, q28D) were tested in 3 histology-based cohorts with final results presented here.
Methods: Pts with rel/ref lymphomas having received > 1 cytotoxic regimen were eligible. Other criteria included ANC > 1000/uL, platelets > 75,000/uL, and normal renal and hepatic function. Pts were enrolled into 3 cohorts: DLBCL (cohort A), FL (cohort B), and other lymphomas (cohort C). CLL/SLL was excluded due to poor single-agent TEM activity. TEM was administered at 25 mg IV weekly, and LEN at 20 mg PO daily (D1-21, q28D). Pts received therapy for up to 1 year, or until disease progression or development of toxicities requiring treatment cessation. All pts received ASA prophylaxis. Response assessments were performed after cycle 2, and every 3 months thereafter.
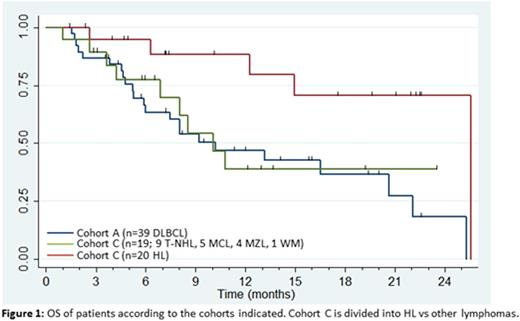
Results: 93 pts (31 female, 62 male), mean age 57 years (range, 23-78 years) were enrolled onto cohort A (n=39 DLBCL), cohort B (n=15 FL), or cohort C (n=39; 20 HL, 9 T-NHL, 5 MCL, 4 MZL, 1 WM). The median number of prior treatments was 4 (range, 1-14). 28 pts had relapsed following autologous stem cell transplantation (ASCT). The median number of cycles delivered was 4 (range, 1-12). Grade 3 or higher non-hematologic adverse events (AE) were uncommon with only fatigue and hypokalemia occurring in >10% of pts; ≥ grade 3 hematologic toxicity included anemia (n=27), lymphopenia (n=40), neutropenia (n=44) and thrombocytopenia (n=42). Three grade 5 AEs were observed (colonic perforation, myocardial infarction and sepsis). The overall response rates (ORR) were 25.6% (5 CR; 5 PR), 46.6% (5 CR; 2 PR), and 64.1% (7 CR; 18 PR) for pts in cohorts A, B, and C, respectively. Of note, cohort B (FL) was closed prematurely due to poor accrual. Median progression-free survival (PFS) for pts in cohorts A and C was 6.0 months (95% CI 3.1 - 8.0 months), and 7.3 months (4.2 - 10.8 months), and median duration of response (DOR) was 11.3 months (2.6 months - not reached) and 5.5 months (2.3 months - not reached), respectively. Median overall survival (OS) for pts in cohorts A and C was 10.2 months (5.9 - 20.6 months), 25.5 months (10.1 - not reached), respectively. Among 20 heavily-treated HL pts in cohort C (median prior treatments 6, range 3-14), all of whom progressed on brentuximab vedotin (BV), and 13 of whom had received a prior ASCT, the ORR was 80% (7 CR; 9 PR), and the median PFS, DOR and OS was 9.2 months, 8.1 months, and 25.5 months, respectively.
Conclusions: The combination of TEM/LEN therapy was well-tolerated and showed encouraging activity in pts with rel/ref lymphomas. The most significant activity was observed in rel/ref HL, with an 80% response rate, including in pts with prior BV exposure and in those relapsed after ASCT. For DLBCL, the addition of LEN did not improve ORR compared to historical experience with single-agent TEM, but we note a provocative prolongation of both PFS and DOR for this otherwise aggressive disease. Further evaluation of mTOR pathway inhibition and immunomodulatory agents in relapsed HL patients is warranted based on these results.
This study was conducted through the University of Chicago Phase II Consortium, supported by NCI contract N01-CM-2011-00071C
Kline:Vasculox: Research Funding; Merck: Honoraria, Research Funding. Petrich:AbbVie: Employment. Rao:Novocure: Consultancy. Smith:TGTX: Consultancy; Juno: Consultancy; Portola: Consultancy; Amgen: Other: Educational lecture to sales force; Genentech: Consultancy, Other: on a DSMB for two trials ; Pharmacyclics: Consultancy; Celgene: Consultancy; Gilead: Consultancy; AbbVie: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal