Abstract
Introduction. HIV infected individuals have an increased risk of developing lymphoma even in the era of combined antiretroviral therapy. Galectin-1 (Gal-1) is known to promote various immunomodulatory functions, including Treg expansion (Dalotto-Moreno et al, Cancer Res 2013), promotion of tolerogenic dendritic cells (Ilarregui et al, Nat Immunol 2009) and apoptosis of fully-differentiated effector T-cells (Toscano et al, Nat Immunol 2007). In the context of cancer, Gal-1 is expressed on both tumor cells and cells in the tumor microenvironment, and is usually associated with immune privilege, tumor escape and hypoxia-driven angiogenesis (Juszczynski et al, Proc Natl Acad Sci 2007; Cedeno-Laurent et al, Blood 2012). Previously, high intratumoral Gal-1 levels have been suggested as an unfavorable outcome predictor in patients with classical Hodgkin lymphoma (cHL) (Kamper et al, Blood 2011). Furthermore, several in vitro studies revealed the benefit of Gal-1 inhibition with regards to overcoming treatment resistance e.g., after anti-VEGF and anti-CD20 therapy (Croci et al, Cell 2014; Lykken et al, Blood 2016). Thus, Gal-1 inhibition may prospectively be an important tool in lymphoma treatment. In this study, we have investigated the Gal-1 expression in pre-therapeutic tumoral tissue samples from patients with HIV-associated lymphoma and its correlation to clinicopathological features at lymphoma diagnosis.
Methods. Adequate pre-treatment formalin-fixed paraffin embedded samples from 40 HIV-positive lymphoma patients were included in a tissue micro array. The study samples were immunohistochemically characterized by a panel of monoclonal antibodies including CD3, CD4, CD8, CD10, CD20, CD79a (MRQ-48), CD30 (Ber-H2) and MUM1, granzyme B, CD68 and CD163. Epstein-Barr virus (EBV) proteins were visualized by latent membrane protein 1 and Epstein-Barr nuclear protein 2. Expression of EBV-encoded smallRNAs was analyzed by in-situ hybridization. Immunohistochemical cell-of-origin (COO) evaluation was carried out according to the Hans classifier. Gal-1 expression was digitally quantified as an area fraction (AF) of the total core area. Estimates of differences between groups were evaluated using Students t test, Pearsons χ2, Fisher's exact test or Spearman correlation where appropriate. Optimal cut-off values of the AF were established by a ROC analysis and calculated using Youden's index for diffuse large B-cell lymphoma (DLBCL) (n=22). Outcome was estimated by Kaplan-Meier time-to-event analyses and compared using the log-rank test. Independent prognostic values were tested by Cox-regression analysis in a multivariate model for factors showing a crude association with p<0.1and proportional hazards between groups.
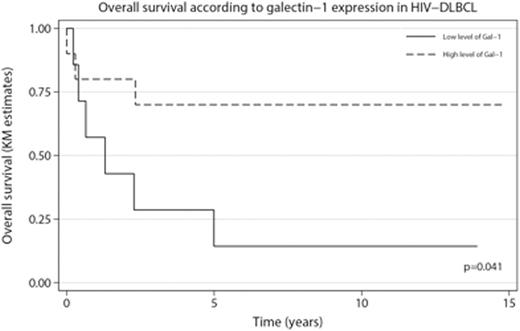
Results. High intratumoral Gal-1 expression in HIV-associated DLBCL tissue correlated with improved outcome (p=0.041), Figure 1. Patients with high Gal-1 expression had a higher occurrence of nodal disease and B-symptoms (p=0.006). Gal-1 expression did not vary according to tumoral EBV status, latency type, International prognostic index (IPI), clinical stage or COO signature. In multivariate analysis adjusted for rituximab treatment, both Gal-1 expression and IPI retained independent prognostic value. Furthermore, intratumoral Gal-1 expression correlated positively with a Th1-signature of the tumor microenvironment including the macrophage marker CD68 (p<0.001), markers of cytotoxic T-cells such as CD8 (p=0.027) and granzyme B (p<0.001), together with the activation marker CD30 (p=0.006). Markers of a Th2-associated microenvironmental signature had no correlation with Gal-1 expression, except for CD163 (p<0.01) that also significantly correlated with CD68 expression (p<0.01).
Conclusion. In HIV-associated DLBCL, intratumoral Gal-1 expression positively correlated with a Th1-signature of the tumor microenvironment. In addition, high pre-therapeutic intratumoral Gal-1 expression was found to be an independent predictor of improved outcome in HIV-associated DLBCL. Interestingly, this is the reverse of our previous findings in non-overtly immunocompromised patients with cHL (Kamper et al, Blood 2011). Further investigations on the potential clinical application of intratumoral Gal-1 expression as a predictive marker in different lymphoma types of the immunocompromised vs immunocompetent host are warranted.
d'Amore:CTI LIfe Sciences: Honoraria, Other: Advisory Boards; Servier: Honoraria, Other: Advisory Boards.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal