Abstract
Activation-induced cell death mediated by Fas/Fas ligand (FasL) system plays a key role in regulating immune response. Although normal NK cells employ this system for their homeostasis, malignant NK cells derived from extranodal NK/T-cell lymphoma, nasal type (ENKL) seem to disrupt the process. ENKL cells usually express both Fas andFasL, whereas they seldom undergo apoptosis. Previous studies showed that loss-of-function mutations of Fas gene are considered a major mechanism of resistance to Fas-mediated apoptosis in ENKL (Shen et al., Am J Pathol 2002; Takakuwa et al., Oncogene 2002). In addition, recent findings indicated that the long form of cellular FLIP (c-FLIPL) is a crucial modulator of procaspase-8 and has the potency to interfere with the Fas-mediated apoptotic pathway in several cancer cells (reviewed by Lavrik and Krammer, Cell Death Differ 2012). We focused on c-FLIPL and examined whether down-regulation of c-FLIPL leads to efficient cell death in ENKL-derived cells.
We used two ENKL-derived cell lines, NK-YS and Hank1, and Fas-sensitive Jurkat as a control. To determine the expression of Fas and FasL, flow cytometry (FCM) was performed and showed the co-expression Fas and FasL in both NK-cell lines. Western blot analysis detected the components of the death-inducing signaling complex (DISC), including FADD, procaspase-8, and c-FLIPL in the three lines. We next confirmed that NK-YS and Hank1 were resistant to the apoptotic stimuli induced by direct Fas cross-linking. Annexin V staining assay by FCM detected that above 40% of Jurkat cells were positive for annexin V, whereas most NK-YS and Hank1 cells failed to show apoptotic changes after Fas ligation.
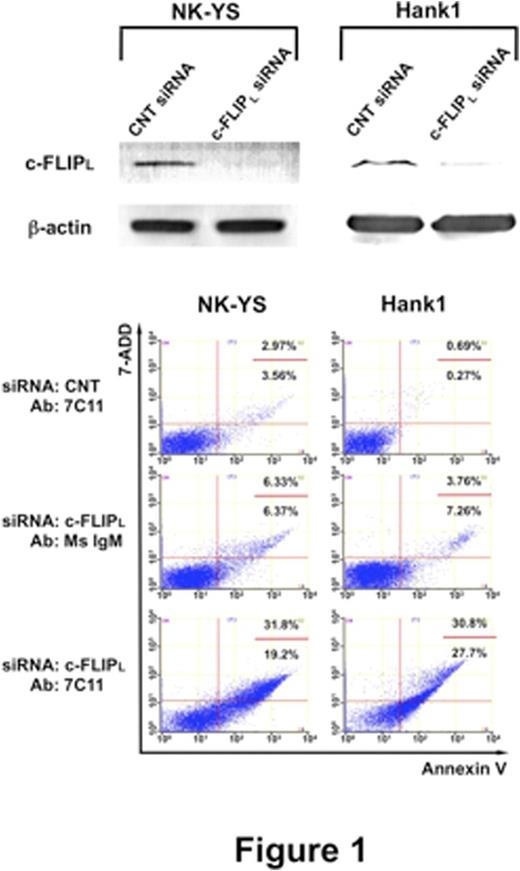
To determine whether Fas-mediated signaling is functional, we investigated the DISC formation in the NK-cell lines. After Fas ligation, FADD and cleaved caspase-8 were coimmunoprecipitated with Fas in NK-YS and Hank1 as well as in Jurkat. Confocal fluorescence imaging also detected fusion signals showing colocalization between Fas and caspase-8 in the three lines. In contrast, cleaved c-FLIPL was coimmunoprecipitated only in NK-YS and Hank1. Confocal microscopy detected that the colocalization of Fas and c-FLIPL was significantly increased in the NK-cell lines, compared with Jurkat. These results showed that c-FLIPL plays a critical role in inhibition of the proximal Fas-mediated signaling pathway in NK-YS and Hank1. Therefore, we next performed RNA interference for c-FLIPL in the NK-cell lines. Although the cells transfected control siRNA hardly showed apoptotic change even after Fas ligation, knockdown of c-FLIPL slightly increased apoptosis in NK-YS and Hank1 (Figure 1). Furthermore, the suppression of c-FLIPL remarkably sensitized the NK cell lines to the Fas stimulus. FCM detected that above 50% of cells came to be positive for annexin V in both NK-cell lines (Figure 1). Here, we demonstrated another mechanism of resistance to Fas-mediated apoptosis in ENKL cells.
Our findings indicate that two ENKL-derived cell lines are similar to activated normal NK cells in being primed to undergo Fas-mediated apoptosis. However, they constitutively express and exploit c-FLIPL, which prevents their Fas-mediated apoptosis. The specific down-regulation of c-FLIPL can induce the autonomous death of ENKL cells. c-FLIPL may be a therapeutic target against ENKL.
Komatsu:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Shire: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal