Abstract
Background: Pediatric Burkitt Lymphoma (PBL) represents the most common malignancy in childhood and adolescent non-Hodgkin Lymphoma (NHL) but occurs in less than 5% of adult NHL (Hochberg/Cairo et al, BJH 2009; Miles/Cairo, BJH. 2012). Mature B-NHL, including Burkitt lymphoma (BL) and primary mediastinal large B cell lymphoma (PMBL) express CD79b+ and have an excellent prognosis with chemo-immunotherapy (Cairo et al Blood. 2007,Goldman/Cairo et al. Leukemia, 2013, Gerrard/Cairo et al. Blood. 2013). The prognosis of PBL has significantly improved over 30 years through the use of short and intense multi-agent chemotherapy, however, a subset of patients with relapsed/refractory mature B-NHL has chemoimmunotherapy resistant disease and a dismal prognosis (≤ 20% 5 years, EFS,Cairo et al.Blood. 2007; Cairo et al. JCO.2012, Miles/Cairo et al. BJH. 2012). It is therefore critical to investigate and to develop targeted translational strategies in BL/PMBL in order to reduce acute morbidities, decrease late effects, and provide new options for those with recurrent disease.
The anti-CD79b-vc-MMAE antibody drug conjugates (ADC, Polatuzumab Vedotin, PV) has demonstrated significant preclinical activity against indolent CD79b+NHL (Polson et. al.Can. Res. 2009; Dornan et al. Blood. 2009). More recently PV has been safe and well tolerated in adults with CD79b refractory NHL (Palanca-Wessels et al. Lancet Oncol.2015), However, PV preclinical activity against mature BL/PMBL is unknown.
Objective: To determine the efficacy of the ADC (anti-CD79b-vc-MMAE) against CD79b+ PMBL in-vitro and rituximab (RTX) sensitive/resistant BL tumor cell lines in-vitro/ in-vivo.
Methods: Raji/Raji4RH (provided by M. Barth, Roswell Park Cancer Institute) and PMBL: Karpas1106P and MedB-1 were cultured in RPMI with 10 or 20% FBS. Tumor cells were incubated with hu anti- CD79b-vc-MMAE, and/or anti-CD79b, MMAE or huIgG1 (generously supplied by Genentech Inc.) for 24 hrs. Cell death was evaluated by staining with annexin V/7AAD and cell proliferation was analyzed by alamar blue by flow-cytometry, n=3. Six to 8 week old female NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ), were divided into 5 groups: PBS only (control), isotype control (IgG), PV (5mg/kg), anti-CD79B mAb (5 mg/kg) and MMAE (5 mg/kg). Mice were xenografted with intravenous injections of Luc+ Raji and Raji4RH cells at 5x106 tumor cells/mouse as we have previously demonstrated (Awasthi/Cairo et al, BJH, 2015). Mice were treated twice a week for 6 weeks. Tumor burden was monitored by IVIS spectrum system.
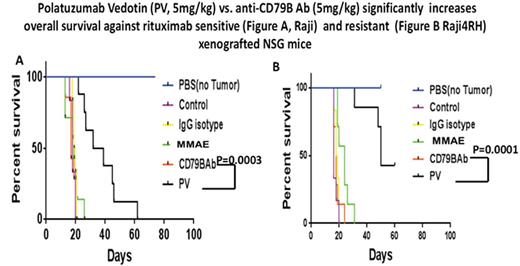
Results: Hu- anti -CD79b-vc-MMAE compared to hu anti-CD79b Ab or control hu IgG1 Ab alone (10µg/ml, 24hrs), significantly enhanced cell death ( apoptosis) in Raji, 47.2±1.3% vs 29.1±6.0% vs. 28.2±4.3%, (p=0.0008 and p=0.00006), Raji4RH, 29.8±9.1% vs 25.4±3.9% vs. 18.0±8.2% (p=NS and p=0.03), Karpas1106P, 46.8±5.3% vs 33.8±3.5% vs. 26.2±0.4% (p=0.02 and 0.006) and MedB-1, 47.4±2.2% vs 27.6±2.4% vs. 23.9±1.7% (p=0.002 and 0.0001), respectively. Hu anti- CD79b-vc-MMAE also significantly reduced cell proliferation compared to hu anti- CD79b Ab or control hu anti -IgG1 Ab alone (20µg/ml, 24hrs). Raji, 56.1±5.1% vs 38.1±0.7% vs. 14.8±0.4% (p=0.001 and p=0.0008), Raji4RH, 53.4±5.4% vs 23.4±5.51% vs. 11.3±0.8% (p=0.004 and 0.006), Karpas1106P, 46.4±0.3 %vs 29.0±1.5% vs. 15.9±0.6% (p=0.005 and 0.00007) and MedB-1, 47.2±7.5% vs 27.7±8.5% vs. 12.3±0.5% (p=0.01 and p=0.0005), respectively. Further, median survival time in mice receiving 5 mg/kg of PV was significantly increased when compared to mice receiving 5 mg/kg of anti-CD79b antibody or IgG isotype control in Raji and Raji4RH ( 35.5 vs.17 vs. 19.5 days, p=0.0001, 0.0003 and 50 vs. 18 vs. 18.5 days, p=0.0001, 0.0001, respectively, Figure. A and B)
Conclusion: Our preliminary data indicates thathu anti- CD79b-vc-MMAE significantly enhances cell death and reduced cell proliferation in RTX sensitive/ and resistant CD79b+ BL and PMBL compared to CD79b Ab or isotype control IgG1 Ab alone. Furthermore, PV significantly increased survival in BL (RTX sensitive/ resistant) NSG xenografts compared to an equal dose of anti-CD79b alone.
Cairo:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal