Abstract
Background: AML remains a disease diagnosed in the aging population with chemotherapy followed by bone marrow transplant in some cases being the standard of care. Although response rates remain around 50-60%, treatment related mortality and disease relapse remain high. Adoptive immunotherapy, especially those targeting T cell co-inhibitory receptors, has proven successful in solid malignancies however, AML remains less explored. Our laboratory has previously demonstrated the feasibility to generate autologous AML reactive T cells in vitro (Mehta/Szabolcs; Immunotherapy 2016). It was noted that "resistant" AML blasts over expressed a number of genes associated with immunosuppressive characteristics. Over expression of these genes may induce T cell functional exhaustion. Therefore, we hypothesized that blocking PD-1 and/or CTLA-4 during co-culture with IFNg activated AML blasts, may enhance T cell activation and cytotoxicity. To test this hypothesis, we tested CTL responses against AML blasts and IFNg ELISpot formation after blocking with PD-1, CTLA-4 or both receptors, and compared the response in untreated T cells. Gene expression profiles of co-stimulatory/co-inhibitory receptors were also monitored to test for correlation.
Methods: We evaluated 12 patients with newly diagnosed AML under an IRB approved protocol with written informed consent of patients. Mononuclear cell preparation was generated from fresh marrow samples or drawn from a biorepository of previously cryopreserved leukophereses. T cells were then purified using immunomagnetic CD3/CD28 beads (Life technologies) and cultivated in media with IL-2 and IL-7 for 2 weeks. AML blasts were cultured over a supporting layer of mesenchymal stromal cells (MSCs) derived from healthy BM donors for 1 week and then cryopreserved. T cells were then co-cultured with restored and irradiated autologous AML cells at an effector: target (E: T) ratio of 5:1 to 40:1. AML and T cells were co-cultured in the presence of Ipilimumab (anti-CTLA-4), or Nivolumab (anti-PD-1), or a combination of both drugs. T cells and AML were re stimulated in X-vivo 15 with IL-12, IL-15 and IL-2 weekly x 3weeks. T cell response to AML was quantitated by IFNg ELISpot assay and Europium TDA (EuTDA) CTL assays independently. Co-stimulatory/co-inhibitory expression on T cells was examined with RT-q PCR assay. Paired-sample student t test was used for statistical analysis with p<0.05.
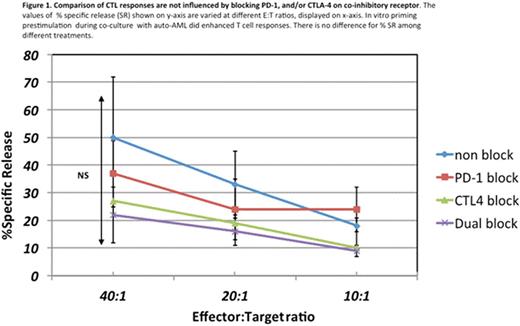
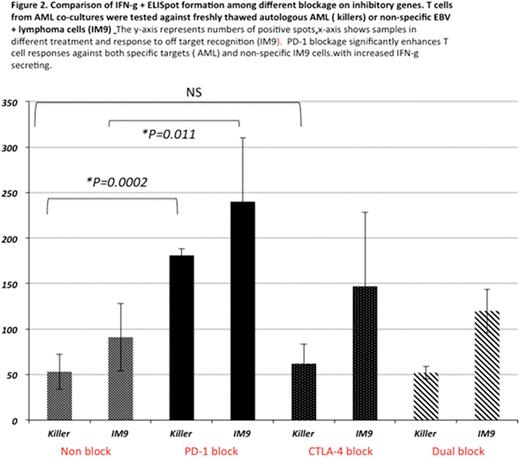
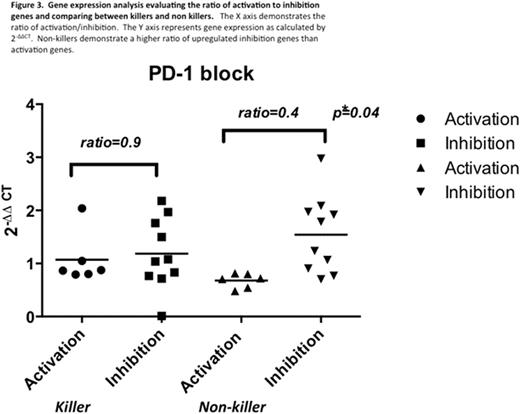
Results and Discussion: Out of 12 samples, 10 (83%) yielded viable AML cells available for cytotoxicity assay. One third (33%) of co-cultures exhibited a positive T cell response in CTL assays ("killers"). There was no difference in CTL activity by blockade of either PD-1 or CTL-4 (Fig 1). IFN-ɣ spot formation in ELISpot was observed in 4/10 samples (40%) with statistical significance noted in cells blocked with PD-1 as compared to all other blockade types (Fig 2). The results indicated that in vitro priming with autologous AML blasts or together with blocking PD-1 can enhance T cell response in 33-40%. By gene expression analysis, the ratio of co-stimulatory to co-inhibitory genes was calculated. In PD-1 blocked cells, the ratio of activation/inhibition was not impacted in T cells from "killers" (0.9; p=0.1), however, T cells from "non-killer cells" had a diminished ratio due to higher expression of co-inhibitory molecules (0.4; p=0.04) (Fig 3). This trend was also present in CTLA-4 blocked cells (0.85; p=0.4 in killers vs 0.54; p=0.03 in non-killers) (data not shown). Interestingly, dual blockage failed to influence gene expression ratio, data not shown.
Conclusion: The above studies demonstrate that cytotoxicity can be achieved in T cells when primed against autologous AML. PD-1 blockade can enhance IFNg production and cytotoxic responses, but CTLA-4 and dual blockade failed to enhance T cell function. The upregulation of an inhibitory pattern of genes in T cells that did not express cytotoxicity (non-killers) could allude to an "inhibitory phenotype" that may be resistant to immunotherapy drug blockade and requires further study.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal