Abstract
Introduction: Therapeutic agents approved by the FDA are required to undergo postmarketing safety surveillance to meet regulatory and contractual requirements. Arsenic trioxide (Trisenox®) is approved for the treatment of patients with acute promyelocytic leukemia (APL) in the second-line setting, but is also commonly used in first-line regimens in combination with all-trans retinoic acid. Here, we summarize the accumulated safety data from global postmarketing surveillance of arsenic trioxide since its initial approval in 2000 to better understand its long-term safety in patients with APL.
Methods: Cumulative data were collected from patients with APL since initial approval in 2000 from spontaneous reports submitted by healthcare professionals and consumers. The data collected were housed in the Teva Pharmaceuticals safety database. Adverse events (AEs) were coded according to MedDRA terms and classified by seriousness and expectedness (unexpected AEs are those not included in the product label at the time of the report or those that occurred at a higher incidence than previously reported).
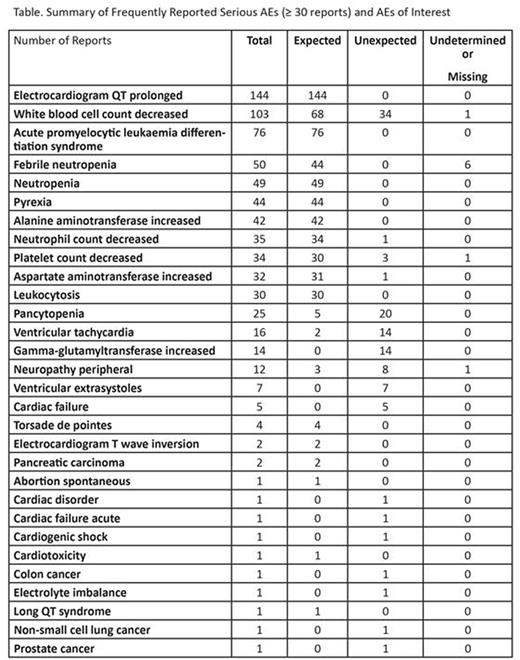
Results: As of July 7, 2016, a total of 2197 AE reports were received in association with arsenic trioxide use in patients with APL. There were 1735 reports classified as serious and 462 classified as nonserious. The most frequently reported (≥ 30 reports) serious AEs and serious AEs of interest are summarized in the table. Serious QT prolongation was the most commonly reported event, but the majority of events were expected, including four cases of torsades de pointes that may be associated with prolonged QT. Serious hematologic toxicities, including neutropenia and leukocytosis, were also common and expected, as was liver toxicity. The most common serious and unexpected events reported included white blood cell decrease, pancytopenia, increased gamma-glutamyltransferase level, and cardiac disorders (primarily ventricular tachycardia, ventricular extrasystoles) that may be associated with prolonged QT in some patients.
Conclusions: The overall safety profile of arsenic trioxide is consistent with known toxicities; the majority of serious AEs are associated with QT prolongation. The clinical significance of the reported QT events is unclear, and reporting rates may change in the future because of evolving criteria for QT assessments (Roboz GJ, et al. J Clin Oncol 2014;32(33):3723). This overview was limited by voluntary reporting. However, the long-term, real-world experience with arsenic trioxide administration as a single agent or in combination provides a broader understanding of its safety profile.
Sponsor: Teva Branded Pharmaceutical Products R&D
Lo-Coco:Teva, Novartis, Baxalta, Pfizer: Consultancy; Teva, Lundbeck: Honoraria, Speakers Bureau. Pathak:Teva Pharmaceuticals: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal