Abstract
In the initial CALGB study and the retrospective analysis that followed 4 years later, high dose cytarabine, used as postremission chemotherapy in patients with acute myeloid leukemia (AML), resulted in significantly better overall survival and sustained remission when compared to lower doses of cytarabine, especially in young patients with core binding factor mutations (CBF). However, the toxicity seen with high dose cytarabine (HiDAC) prompted several studies to evaluate the role of intermediate dose cytarabine (MidAc). These studies concluded that MidAc produced similar overall survival and leukemia free survival to HiDAC while achieving a better side effect profile. Following these later findings, the consolidation protocol for AML patients 60 years or younger treated in the Singapore General Hospital was changed to 3 - 4 cycles of cytarabine 1.5g/m2 q12 D1, 3, and 5 (MidAc) from 3g/m2 q12 D1, 3, and 5 (HiDAC) in 2011. Cytarabine dose for older patients remain unchanged at 1g/m2 q12 D1, 3, and 5. A five year landmark analysis was performed to assess the difference in outcomes between these two regimens.
Patients with non-M3 AML treated between 2004 and 2015 who received a cytarabine consolidation after achieving complete morphologic remission post standard 3+7 induction procotol (idarubicin 12mg/m2 D1-3 and cytarabine 100mg/m2 as a continuous infusion from D1-7) were included in the analysis. Patients who received an autologous or allogeneic hematopoietic stem cell transplant at first complete remission were excluded.
92 patients were identified for analysis with median age of 47 (range 16-76). The majority of patients belonged to the ELN favorable risk group (62 out of 92). 77 patients were aged 60 years or younger amongst which 48 patients received HiDAC and 29 patients received MidAc after a change in protocol. The median follow up for patients receiving HiDAC and MidAc was 104 and 39 months respectively.
The 5-year overall survival (OS) between patients who had HiDAC and MidAc was 66.7% and 45.7% (p=0.16); and the 5-year leukemia free survival (LFS) was 51.9% and 41.6% (p=0.23). For comparison, the 5-year OS for patients above the age of 60 treated before and after 2011 was 16.7% and 46.7% (p = 0.3) and LFS was 16.7% and 55.6% (p= 0.35) respectively.
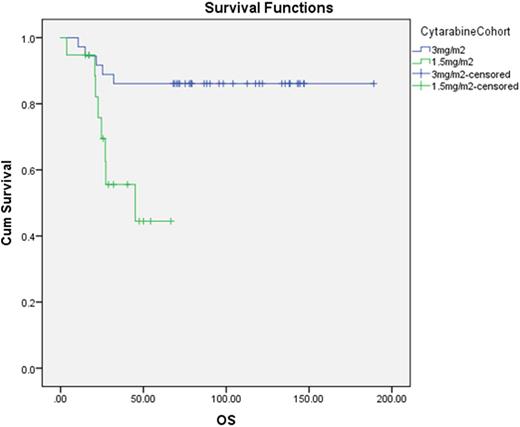
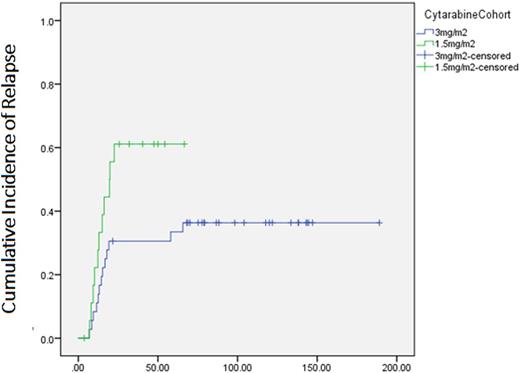
Amongst 55 patients aged 60 or younger with favorable ELN risk, the survival outcomes were significantly better in the cohort receiving HiDAC compared with MidAc. 5 year OS were 86.1% vs. 44.5%, median OS not reached vs. 45 mths (p= 0.004). 5 year LFS were 63.9 vs. 36.8%, median LFS not reached vs. 20mths (p= 0.03). The cumulative incidence of relapse at 5 years was 33.4% in HiDAC and 61.1% in MidAc (p=0.04). No survival differences between the HiDAC and MidAc cohort were seen in the non-favorable risk group; 5 year OS was 26.7% vs 36.7% (p = 0.22), 5 year LFS was 26.7% vs 33.3%(p = 0.78) respectively.
13 (23.7%) favorable risk patients aged 60 or younger died during the follow up period. 5 patients died of complications of treatment, 4 (8%) in the HiDAC cohort and 1(3%) in the MidAc group (p= 0.4). Most deaths (4 out of 5) were attributable to neutropenic sepsis. Median age of patients who died during treatment was 54 years (range: 36 to 58) compared to 45 years (range: 15 to 60), (p = 0.23) amongst those who did not die from treatment related complications.
In conclusion, in our cohort of patients consolidated with cytarabine monotherapy, HiDAC offers a better OS and LFS outcomes compared with MidAc in younger patients with favorable risk profile, albeit with a non-significant trend to an increase in treatment related mortality. Further studies looking into the interaction of cytarabine dose with specific genetic and cytogenetic AML sub-groups are warranted.
OS of patients aged 60 or younger with ELN favorable risk AML (p= 0.01)
Cumulative Incidence of Relapse of patients aged 60 or younger with ELN favorable risk AML (p= 0.04)
Hwang:Roche: Honoraria, Other: Travel support; Celgene: Honoraria, Other: Travel support; Pfizer: Honoraria, Other: Travel support; Sanofi: Honoraria, Other: Travel support; Janssen: Honoraria, Other: Travel support; MSD: Honoraria, Other: Travel support; Novartis: Honoraria, Other: Travel support; BMS: Honoraria, Other: Travel support.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal