Abstract
Intermediate dose cytarabine (IDAC) defined by daily intravenous bolus of 1g/m² during 5 days has been recently defined as the standard control arm in phase 3 placebo-controlled randomized trials for patients with relapsed or refractory acute myeloid leukemia (R/R AML). In these trials assessing clofarabine/IDAC (CLASSIC-1 study, Faderl S et al., JCO 2012) or vosaroxin/IDAC (VALOR study, Ravandi F. et al., Lancet Oncol 2015) vs placebo/IDAC, complete remission rates and median overall survival with placebo/IDAC were 17.8%/18.9% and 6.3/6.1 months in the CLASSIC-1 and VALOR studies, respectively. However, the dose-intensity of this IDAC regimen remains questioned in routine practice since many centers still use higher doses of cytarabine often in combination with an anthracycline and a third drug including fludarabine, etoposide or gemtuzumab ozogamycin (FLAG-ida, MEC or MIDAM regimen for example) although these regimen have proved little efficacy and higher toxicity. We assessed the outcome of R/R AML patients that fulfilled main VALOR inclusion criteria consecutively treated in our center with intensive salvage regimen.
All patients with a diagnosis of AML in first relapse or with refractory disease were eligible for this study. Acute promyelocytic leukemia were excluded. Relapse was defined as re-emergence of at least 5% leukemia blasts in bone marrow or at least 1% blasts in peripheral blood 90 days to 24 months after first complete remission or complete remission with incomplete platelet recovery. Refractory AML was defined as persistent disease at least 28 days after initiation of induction therapy, or relapse less than 90 days after first complete remission (CR) or CR with incomplete platelet recovery (CRi). All patients have received previous induction therapy with an anthracycline. Salvage regimen used were mainly cytarabine 3 g/m²/12h, d1-4 plus idarubicine 12 mg/m²/d, d1-3 or dauno 60 mg/m²/d, d1-2 or amsacrine 200 mg/m²/d, d1-3; less frequently MiDAM (mitoxantrone 12mg/m² d1-3, cytarabine1 g/m²/12h d1-5, GO 4-6mg/m², d4) or FLAG-Ida. Cytarabine dose for patients >60 was reduced to 1g/m²/12h, d1-5.
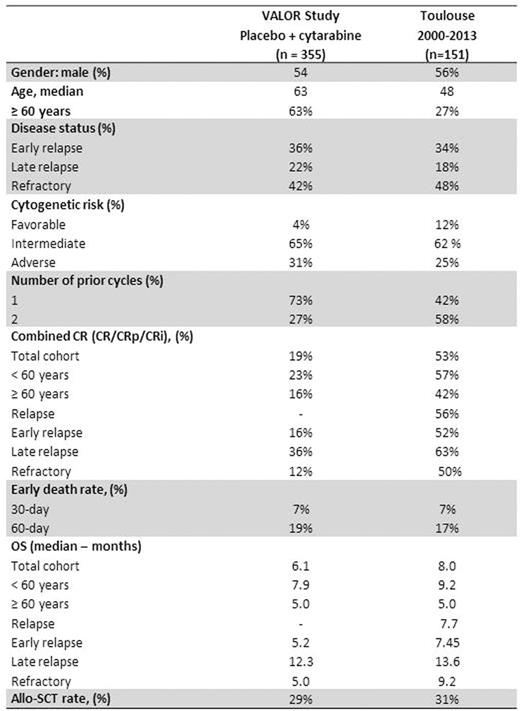
We found, in our database, 151 R/R AML according to VALOR criteria treated between 2000 and 2013: 72 patients (48%) had refractory diseases (primary refractory: n=60, 40% and relapse less than 90 days after CR: n=12, 8.0%) and 79 (52%) had relapsed (early relapse more than 90 days after CR and less than one year: n=52, 66%; late relapse between one and two years: n=27, 34%). Patients characteristics were as follows: 85 (56%) were male, median age was 48 years (interquartile range [IQR], 36-60.5; 38 (27%) were 60 years or older). Cytogenetics at diagnosis was favorable in 18 (12%); intermediate in 93 (62%); adverse in 38 (25%) or unknown in 2 (1%) patients, respectively. They were treated as first line therapy with one (42%) or two cycles (58%). Early death rates at day 30 and day 60 were 7% and 17% in the whole cohort; 6% and 12% in younger patients and 12% and 29% in patients 60 years or older. Combined CR rate (defined as CR and CRi) was 53% for the whole cohort, and 57%/42%/56%/52%/63%/50% for <60 years/≥60 years/relapses/early relapses/late relapses/refractory diseases respectively. Allogeneic-SCT was performed in 25 out of 80 patients (31%) having achieved CR after salvage treatment. Median overall survival (OS) was 8 months for the whole cohort and 9.2/5.0/7.7/7.5/13.6/9.2 months for <60 years/≥60 years/relapses/early relapses/late relapses/refractory diseases respectively.
Using VALOR inclusion criteria, age is the main factor that discriminate R/R AML patients selected for clinical trials using IDAC as control arm compared to patients of daily practice. Thus, those patient populations remain hardly comparable. However, as far as CR is concerned, IDAC seems suboptimal as compared to higher-intensity regimen used in daily practice. Yet, this higher CR rates do not translate into a significant gain in survival since median OS remains close to that of IDAC with the possible exception of refractory patients who seem to benefit from higher-intensity regimen. Thus, although unsatisfactory, IDAC could be considered as a fair control arm for overall survival endpoint especially in patients older than 60 year of age.
Tavitian:Novartis: Membership on an entity's Board of Directors or advisory committees. Huguet:Pfizer, Novartis, BMS, Ariad, Jazz, Amgen: Membership on an entity's Board of Directors or advisory committees. Recher:Celgene, Sunesis, Amgen, Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene, Sunesis, Amgen, Novartis, Chugai: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal