Abstract
Background: Acute myeloid leukemia (AML) induction traditionally includes seven days of cytarabine and three days of an anthracycline (7+3). Co-administration of cladribine may increase efficacy of cytarabine. This rationale directed Saint Louis University (SLU) to adopt a regimen derived from the Polish Adult Leukemia Group (Holowiecki, JCO 2012) which adds five days of cladribine to idarubicin based 7+3 (IAC). We report the SLU experience with toxicity and survival following IAC induction. Additionally, we discuss the effects of IAC in patients 60 and older as an area of particular interest.
Methods: Retrospective analysis of patients with newly diagnosed AML (APL excluded) and high-risk MDS who received IAC from January 2009 to September 2015. IAC was initiated as cytarabine 200mg/m2 by continuous infusion for seven days, idarubicin 12mg/m2 daily for three days, and cladribine 5mg/m2 for five days. Mortality and disease response were analyzed, with stratification by age and NCCN leukemia risk classification.
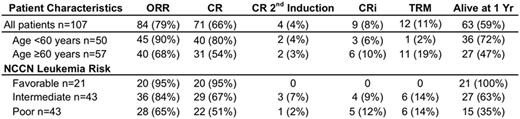
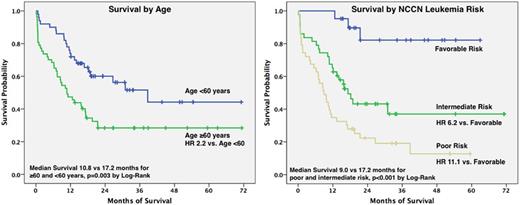
Results: Of 107 patients, 57 patients (53%) were age 60 and older. By NCCN cytogenetic and molecular stratification, 21 (20%) had favorable risk; 43 (40%) intermediate risk; and 43 (40%) poor risk leukemia. Complete response (CR) occurred in 75 (70%) patients and CRi in an additional 9 patients (8%) for an overall response rate of 79% (see Table 1). At one year, 58% of patients were still alive with 47% (27/57) of patients ≥60 years and 72% (36/50) of those <60 years surviving (OR 2.8, 95% CI 1.3-6.4). When stratifying by NCCN risk, no deaths at one year occurred among the favorable risk group (0/21), but 16/43 (37%) and 28/43 (65%) occurred in intermediate and poor-risk strata, respectively (p<0.001 by chi-squared). Median overall survival was 17.2 months with mean follow-up of 32.8 months. Cox proportional hazard ratio (HR) for death were significant for age ≥60 compared to age <60 (HR 2.2, 95% CI 1.3-3.7). Additionally, HR for intermediate risk leukemia was 6.2 (95% CI 1.8-21) and for poor risk leukemia it was 11 (95% CI 3.4-36), when each were compared to favorable risk. Thirty-three (31%) of patients were known to have received an allogeneic transplant, of which 42% (14/33) were 60 and older.
Adverse events: Overall, the treatment related mortality (TRM) (defined as death within the first 28 days) was 11%. Eleven of 12 TRM events occurred in patients ≥60 years (OR 12, 95% CI 1.5-94). Diarrhea occurred in 71% of patients with 46% enduring mucositis. Intensive care unit admission occurred in 23% overall, and infections were documented in 73% of patients. Most common infectious etiology was coagulase negative staph in 29% (23/78). For patients surviving to discharge (90/107), median hospitalization was 30 days post induction.
Conclusions: Cladribine combined with cytarabine and idarubicin is an effective induction regimen for AML with high rates of CR, even in patients 60 and older. Our data show that NCCN leukemia risk is a better predictor of survival compared to age and that patients aged ≥60 years have better survival (lower HR for death) compared to those with intermediate or poor risk leukemia. Additionally, CR and 1 year overall survival with IAC are better than expected in the older cohort, however, TRM is elevated among those 60 and older. Higher intensity regimens in older patients may be justified in select patients, especially given the greater life expectancies enjoyed in the modern era. Several ongoing prospective trials investigating the safety and efficacy of cladribine in older patients will provide additional information to inform the use of IAC in older patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal