Abstract
Background: High-dose cytarabine and mitoxantrone (HAM) have been used as salvage chemotherapy in patients (pts) with relapsed/refractory AML. Mitoxantrone is less cardiotoxic than other anthracyclines and can be tolerated in older pts. Crenolanib is a novel, type I, oral pan-FLT3 inhibitor with promising single-agent activity in multiply relapsed/refractory FLT3+ve AML. Crenolanib has a half-life of 6-8 hrs and does not accumulate after chronic dosing. We here report data from the first 8 pts with primary relapsed or refractory AML treated with HAM followed by crenolanib.
Design: Pts with AML and/or prior MDS regardless of FLT3 status (wild-type or mutant) were enrolled in this study. Eligible pts must have progressed on or be refractory to their first line AML therapy. Pts were treated with HAM (cytarabine 1.0 g/m2/d for d1-6 and mitoxantrone 10 mg/m2 for d1-3) followed by crenolanib 100 mg TID, starting from d8 continuously for a maximum of 49d per cycle (2 cycles of treatment were allowed). Older pts (≥60 yr) or pts who had prior SCT or who required a second induction cycle received lower dose of HAM (cytarabine 0.5 g/m2/d for d1-6 and mitoxantrone 8 mg/m2 for d1-3). Pts in remission following salvage induction were eligible to proceed to allo SCT.
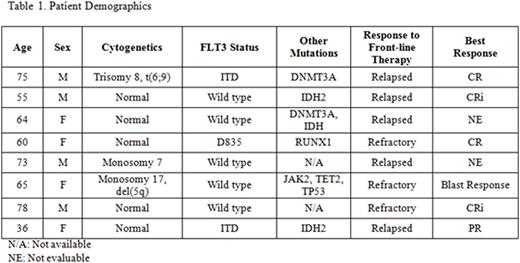
Results: 8 pts (4 males, 4 females) with first relapsed or primary refractory AML have been enrolled and received HAM followed by crenolanib. The median age on study was 64 (range 36-78) with 6/8 pts ≥ 60yr and 3 pts older than 70yr. 3/8 pts came on study with FLT3 activating mutations, including ITD (2 pts) and D835 (1 pt). 7/8 pts had de novo AML, and 1 had prior-MDS after receiving prior chemotherapy for ovarian cancer. 3/8 pts were primary refractory to first line therapy with the remaining 5 pts having relapsed following response to first line therapy. Pt demographics are summarized in Table 1.
8 pts received 1 cycle of salvage treatment with HAM followed by continuous daily crenolanib (100 mg TID). 6 pts were evaluable for responses with a complete remission rate of 67% (2 CR, 2 CRi), including 2 pts who were refractory to front line chemotherapy. 2 of 3 pts with FLT3 activating mutations (1 with ITD and 1 with D835) achieved complete remission with complete count recovery; the third pt (FLT3-ITD) had 10% residual blasts after 1 cycle of induction, and is currently planned for transplant.
Crenolanib with salvage chemotherapy was well-tolerated in all 8 pts administered full dose crenolanib (100 mg TID) following HAM. No crenolanib dose reductions were required in any of the 8 pts during induction; however crenolanib was held for intercurrent illnesses, such as C. difficile diarrhea. 2 events of grade 1 ALT/AST elevation were noted during salvage treatment with HAM, but did not cause delays in the initiation of crenolanib. Moreover, the addition of crenolanib did not cause any further elevation of ALT/AST in these 2 pts and no dose reductions or holds were required. Only 1/8 pts showed an elevation in total bilirubin, this event was a transient grade 2 elevation attributed to concomitant medication for fever and returned to normal within 3-5d with no intervention required.
Conclusions: Full-dose crenolanib was well-tolerated when given sequentially with HAM in an older pt population (median 64 yrs) with primary relapsed/refractory AML. Four pts achieved CR/CRi following 1 cycle of HAM/crenolanib, including 2 pts with WT FLT3 and 2 pts with FLT3 activating mutations (1 ITD, 1 D835). This trial has been expanded to allow combination of full dose crenolanib with other standard salvage chemotherapies, including MEC (mitoxantrone, etoposide, cytarabine) and FLA(G)-IDA (fludarabine, cytarabine, idarubicin w/ or w/o G-CSF). A phase III is initiated combining crenolanib and salvage chemotherapies in first relapse or primary refractory AML pts with FLT3+ve activating mutations.
Iyer:Genentech: Research Funding; Seattle Genetics: Research Funding; BMS: Research Funding; Incyte: Research Funding; Arog: Research Funding; Celgene: Research Funding. Karanes:Arog: Research Funding. Eckardt:Arog: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal