Abstract
Background: The hypomethylating agents (HMAs) azacitidine and decitabine were approved for treatment of myelodysplastic syndromes (MDS) in the United States (US) in 2004 and 2006, respectively, with demonstrated survival benefit for azacitidine among patients with higher risk MDS. Based on accumulating clinical and biologic evidence, chronic myelomonocytic Leukemia (CMML) has been reclassified as a clinical entity distinct from MDS. Despite a lack of evidence regarding a survival benefit associated with use of HMAs in CMML, HMAs are used for management of CMML in the US. Here, we take advantage of a natural experiment -market entry of HMAs- to assess whether HMA use is associated with survival among older adults with CMML.
Methods: In a retrospective cohort study, we used the Surveillance, Epidemiology, and End Results (SEER- Medicare database to identify older adults (≥ 66 years in age) diagnosed with CMML (the International Classification of Diseases for Oncology 3rd edition histology 9945) in the 2001-2011 period, with follow-up through end of 2013. We identified HMA users based on procedure codes from claims, limiting to those who initiated therapy after their CMML diagnosis. We used Kaplan-Meier methods and log-rank test to analyze unadjusted survival. We used propensity score matching (PSM) method to match patients diagnosed during 2007-2011 who received HMAs (Post-HMA-users) with patients diagnosed in 2001-2003 who never received HMAs (Pre-HMA-non-users) during the study period. The covariates used for PSM included age at diagnosis, sex, race, zip code, education, neighborhood income, modified Elixhauser comorbidity count and disability status score (a claims-based surrogate for performance status), hospitalizations for bleeding or infection, and receipt of red blood cell and/or platelet transfusions prior to diagnosis. We did not match HMA users to HMA non-users in the post 2006 era to minimize impact of unobserved variables that might affect the decision to use HMAs. To test the competing hypothesis that survival improved as a result of other treatments/supportive care over the observation period, we also matched and compared CMML patients who never received HMAs in the pre-HMA and post-HMA periods. We used Cox proportional hazards models to assess the change in survival according to HMA use or time period.
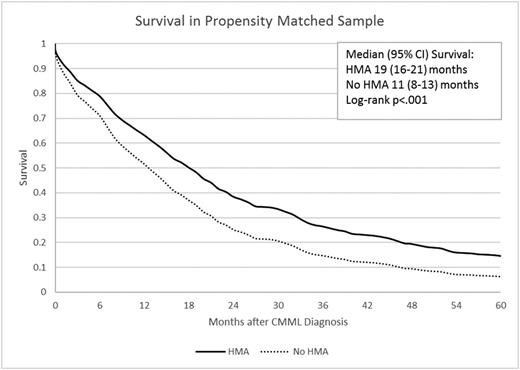
Results: A total of 1,868 individuals with CMML were eligible, of whom 1723 (92.2%) had died during follow-up. The median age at diagnosis was 79 years (interquartile range [IQR]: 73-84), 90.3% were white, and 60.7% were males. Median overall survival (OS) from diagnosis was 13 (95% confidence interval [CI]: 12-14) months. During follow-up, 259 patients (13.9%) received HMAs including 120 patients who received only azacitidine (6.4%), 81 who received only decitabine (4.3%), and 58 patients (3.1%) who received both drugs. Among those who received any HMA therapy, the median duration from diagnosis to first initiation of the HMA was 3 (IQR: 1-11) months. Only 18 patients (1%) underwent allogeneic stem cell transplantation (alloSCT) during follow-up. From diagnosis, the unadjusted median OS of patients who received any HMA during study was 22 (95% CI: 19-25) months compared to 11 (95% CI: 9-12) months for those who never received a HMA during study (p<.001). Among 180 Post-HMA-users and 328 Pre-HMA-non-users who were PS-matched, the median survival was 19 months in Post-HMA-users compared to 11 months in Pre-HMA-non-users (hazard ratio [HR] = 0.49, 95% CI: 0.38-0.64; p<.001, Figure 1). We also PS-matched 290 Pre-HMA-Non-users with 509 post-HMA-non-users and found that the two groups had similar mortality rates (HR = 1.06, 95% CI: 0.87-1.29; p=.55).
Conclusions: In one of largest cohorts reported, we found that only a small percentage (14%) of older patients diagnosed with CMML received HMA therapy. Moreover, only 1% underwent alloSCT, the only potentially curative therapy. Our results suggest that use of HMA is associated with a 51% reduction in risk of death in CMML patients who received HMA after 2006 compared to propensity score matched patients who were diagnosed before 2004 and did not receive HMAs. The association is probably not attributable to potential improvement in other treatments/supportive care over the observation period. Our findings provide strong evidence for a survival benefit associated with the use of HMA in CMML.
Zeidan:Ariad: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Incyte: Consultancy, Honoraria. Huntington:Oncosec Medical: Equity Ownership; Celgene: Consultancy, Honoraria; Exelixis: Equity Ownership; Geron: Equity Ownership; Johnson & Johnson: Consultancy; Pharmacyclics: Honoraria. Podoltsev:Ariad: Consultancy, Honoraria; Incyte: Consultancy, Honoraria. Gore:Celgene: Research Funding. Ma:celgene: Consultancy, Honoraria. Davidoff:PhRMA: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal