Abstract
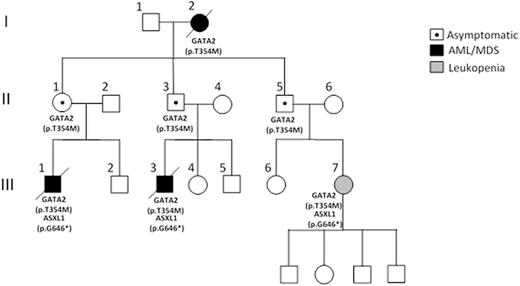
Background: While myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) are considered sporadic hematopoietic stem cell clonal disorders, there are rare occurrences of familial cases (<5%) where two or more individuals within the same family are affected. These high-risk examples are characterised by wide variations in the age of onset, disease latency and outcome between and within families, making their investigation, follow-up and treatment all the more challenging.To date, germline mutations in 11 disease genes have been described, with mutations in the myeloid transcription factor GATA2 representing one of the best-characterised genetic loci predisposing to inherited hematological malignancies. We have noted that within GATA2 families, particularly those segregating a germline p.Thr354Met mutation, there is striking evidence of reduced penetrance. In our example, two first-degree cousins (III.1 and III.3) developed high-risk MDS with monosomy 7 with a third cousin (III.7) presenting with significant leukopenia (monocytopenia [0.1x109/L] and neutropenia [0.8x109/L]). This contrasts with the parental generation (II.1, II.3 and II.5) who all remain hematologically normal and symptom free into their mid-late 60s (Figure 1). We therefore set out to understand these differences in clinical presentation between mutation carriers.
Aims:To investigate the molecular mechanisms underlying the variable penetrance and clinical heterogeneity observed in a GATA2-mutated family.
Results:Targeted deep-sequencing of 33 genes frequently mutated in MDS/AML revealed a low overall burden of acquired mutations in the symptomatic carriers with no mutations detected in asymptomatic family members. It was noteworthy that an acquired ASXL1 mutation (p.Gly646TrpfsTer12) was identical in all affected individuals (III.1, III.3 and III.7) (Figure 1) although the variant allele frequency was lower (12%) in III.7 and remained stable (range 12-6%) over a 4 year monitoring period. GATA2 expression was lower in III.7 as assessed by quantitative RT-PCR and strikingly this was associated with monoallelic expression of the mutated GATA2 allele with complete loss of the wild-type (WT) allele expression. Temporal analysis of III.7 at yearly intervals demonstrated reactivation of the WT allele 2 years later, coinciding with a marked improvement in hematological parameters (normal monocyte count, neutrophils >1x109/L). These changes in GATA2 expression were not linked to gross changes in methylation, as assessed by methylation specific PCR and bisulphite sequencing, nor acquisition of additional mutations in the WT promoter. Instead, we believe that allele-specific fluctuations in expression are accompanied by changes in chromatin structure at the promoter. Using a SNP (rs1806462 [C/A]) located in the 5'UTR of GATA2, we assessed allele-specific enrichment of H3K4me3 and H3K27me3 chromatin marks by chromatin immunoprecipitation. Sanger sequencing revealed a significant enhancement in the deposition of H3K4me3 activating chromatin mark on the mutated allele compared to the WT allele at diagnosis and this was reversed at later follow-up, correlating with reactivation of the WT allele expression. There were no discernible allele-specific differences in the H3K27me3 mark across the phenotypes at different time-points.
Conclusion: Variable penetrance amongst germline mutation carriers is a feature of many families with inherited forms of MDS/AML and this may be related to the nature of secondary genetic events acquired in at-risk individuals. In this study, however, we show that changes in the WT:mutant allele expression ratio as a result of local and allele-specific changes in chromatin deposition may also influence the penetrance of the inherited mutation.
Cavenagh:Amgen: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal