Abstract
Introduction:
Platelet refractoriness due to alloimmunization (AI) is associated with increased bleeding, longer hospitalization, higher costs, and delay of therapy in oncologic patients. Platelet refractoriness in AI patients is traditionally managed by using commercially-available cross-match compatible and/or HLA-matched platelets. Our HLA laboratory has developed a fluorescence cytometric-based XM (FCXM) for patients with severe AI and platelet refractoriness for whom HLA compatible platelets were not available. We present a retrospective analysis of the utility of FCXM in oncologic patients who are refractory to random single donor apheresis platelet transfusions.
Methods:
Patients with platelet refractoriness during 2015 to 2016, as determined by platelet increments <10,000, and having HLA Class I antibodies with a Calculated Panel Reactive Antibody (cPRA) >90%, were selected for study. Data collected include gender, age, diagnosis, ongoing treatment and underlying medical illness during hospitalization, complications, time of release of unit from the Blood Bank, and time of platelet count post-transfusion. Platelet counts increments within 0-1 hour and 1-2 hours after transfusion were recorded. Platelet increments from random-donor platelet units served as controls for FCXM platelet unit increments for the same patient. Statistical analysis was performed using an unpaired t test.
Results:
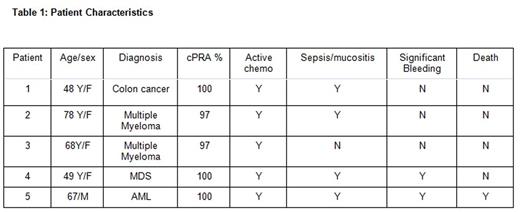
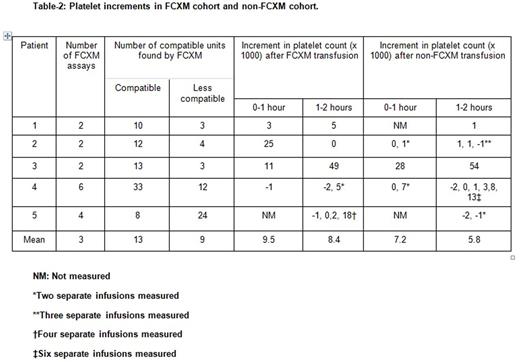
We identified 36 patients with platelet refractoriness, of whom 5 met our study criteria (cPRA >90%): 4 female, 1 male. Median age was 67 years (range 48 to 78), with 4 Caucasians and 1 African American. Underlying diagnoses were multiple myeloma (2), colon cancer (1), acute myelocytic leukemia (1), and myelodysplastic syndrome (1). Two out of 5 patients had significant bleeding, 4 out of 5 had sepsis and mucositis (Table 1). All units were leukocyte-reduced, single donor apheresis platelets. The mean number of FCXM testing performed was 3 (8 units each, 24 total) with 13 and 9 units that were compatible and less compatible identified, respectively. The mean platelet increment within 0-1 and 1-2 hour of transfusion for FCXM vs non-FCXM were 9.5 and 7.2 (p= 0.78), and 8.4 vs 5.8 (p= 0.7), respectively. While a trend existed, the small numbers in the study were insufficient to demonstrate statistical significance (Table 2). One patient died during hospital admission due to diffuse pulmonary hemorrhage post induction chemotherapy. Although 1 other patient had significant bleeding, the remaining four patients were able to complete their planned chemotherapy.
Conclusion:
An overall increase in platelet counts occurs with FCXM identified compatible platelet units in severely AI patients who were refractory to random single donor apheresis platelet units. Although the mean increment using FCXM platelets was < 10,000 and did not differ statistically from the non-FCXM group, we were able to identify units to transfuse patients during chemotherapy and help prevent/treat major bleeding complication and death in 4/5 patients. FCXM testing may be a viable option for identifying compatible platelet units in severely AI patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal