Abstract
Background: Chronic graft versus host disease (CGVHD) remains a notable cause of post transplant morbidity and mortality, limiting quality of life and survival in the allogeneic transplant survivor. CD4+CD25+CD127dimFoxP3+ regulatory T cells (Treg) are a naturally occurring cell population isolatable in the peripheral blood (PB) with key immune regulatory function. Treg have been shown to protect against GVHD in a number of murine models and in patients. Further, clinical correlations have been observed between the presence of CGVHD and a decreased frequency of Treg post allogeneic hematopoietic cell transplant (HCT) as well as the use of low dose IL-2 resulting in clinical responses with an associated increase in Treg. Based on these observations we embarked on a phase I safety and tolerability trial of the treatment of steroid-refractory or dependent CGVHD with infusion of donor Treg in matched related donor recipients.
Methods: Eligible patients were ³ 18 years of age with steroid refractory or dependent CGVHD with a matched related HCT donor. All patients were on systemic immunosuppression at the time of enrollment. Donor derived Treg were enriched from a non-mobilized PB apheresis product over 2 days of apheresis. The fresh apheresis product underwent enrichment by CD25+ immunomagnetic selection on the CliniMACS¨ Cell Selection System (Miltenyi Biotec) and Treg were purified by high-speed flow cytometry and gated on the CD4+CD127dim population. The functional activity of the Treg product was assessed via a mixed lymphocyte reaction (MLR). The trial dosing was through a 3+3 dose escalation design with three Treg dose cohorts: 1 x 10E5 Treg/kg, 5 x 10E5 Treg cells/kg and 1.5 x 10E6 Treg cells/kg.
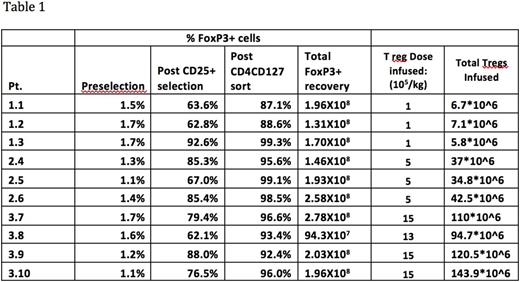
Results: Ten patients were enrolled and received donor Treg cell infusions. Isolation of highly purified Treg was successful from all donors. A median of 1.9x10E8 (range 0.9-2.78x10E8) Treg were collected with a purity that ranged from 87.1-99.3% FoxP3+ (Table 1). Function of the Treg enriched apheresis product confirmed Treg suppressive activity with a median suppression of 69.1% (48.7-77.2%) at a ratio of responder cells:CD4+/CD8+:Treg cells of 1:2:1, with sustained suppression to a ratio of 1:2:0.1. All 10 pateints were treated with three patients treated on all three dose cohorts and an additional patient was treated on the third cohort due to the planned cell dose being not reached with the third patient-donor pair. There were no adverse toxicities with the Treg infusion and no incidence of acute GVHD or acute exacerbation of CGVHD. The ratio of Treg/CD4+ cells in the patients' PB ranged from 2-4.4% at the time of the infusion and remained stable <3 months post infusion. Six of 10 patients have had stable to improved CGVHD with one of these patients having had a flare of CGVHD in the setting of a lapse in immunosuppressive medication and response with reinstitution of immunosuppression. Four patients have had unresponsive or progressive disease requiring the addition of new immunosuppressive agents <2 months post Treg cell infusion. All enrolled patients remain alive at a follow up of >150 days post Treg cell infusion.
Summary: This study is the first clinical experience utilizing highly purified donor derived Treg isolated through clinical scale high speed cell sorting. Donor Treg cell therapy for the treatment of steroid refractory or dependent CGVHD at doses of 1.5x10E5-1.5x10E6 Treg/kg were administered without toxicity including absence of AGVHD or exacerbation of CGVHD. The Treg dose of 1.5 x 10E6/kg of recipient weight may be the maximum feasible dose attainable without ex vivo Treg cell expansion. Treg mediated suppression in the MLR documented the in vitro suppressive capability of the Treg cells with investigations ongoing to characterize the functionality of the Treg cells infused. Although this trial was designed to describe the feasibility, safety and tolerability of Treg cell infusions in patients with active CGVHD, the preliminary outcomes of clinical responses and survival are encouraging and comparable to other therapies for steroid refractory/dependent CGVHD with only a single infusion of cells. Further strategies such as multiple infusions or the addition of low dose IL-2 could be considered to attempt to augment the clinical efficacy of Treg based cellular immunotherapy.
Meyer:Stanford University: Patents & Royalties. Negrin:Stanford University: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal