Hemolytic transfusion reactions (HTRs) occur after donor packed red blood cell (PRBCs) transfusions with antigenic differences including the major antigen system, ABO, as well as minor antigen systems such as Rh, Kell, Duffy, Kidd, JH, and U. These reactions are classified as acute if they occur within the first 24 hours or delayed if occurring after 24 hours. Antigen mismatched transfusions activate the classical pathway of complement leading to intravascular hemolysis via C5b-9 and extravascular hemolysis via C3b mediated phagocytosis in the spleen and liver (Stowell et al. Clin Devel Immunol 2012).In addition to laboratory evidence of hemolysis and increased antibody titers, patients can experience a wide range of clinical symptoms including fever, rash, chest, abdomen or back pain, signs of hemodynamic instability and death. (Strobel. Trans Med & Hemo 2008)
Mouse models have demonstrated that the inhibition of complement receptor 1 resulted in increased survival of mismatched transfused RBCs (Yazdanbakhsh et al. Blood 2003). The terminal complement inhibitor, eculizumab, has been used to successfully rescue a patient after a severe ABO incompatible hemolytic transfusion reaction (Weinstock et al. Transfusion 2015). Based on these data, we postulated that the prophylactic administration of a C1 esterase inhibitor would interrupt activation of the classical complement pathway, mitigating a hemolytic transfusion reaction to antigen mismatched PRBCs.
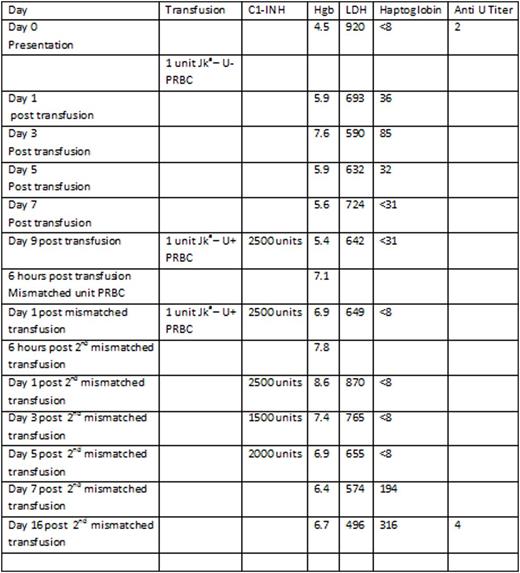
A 50 year old African American male with widely metastatic prostate cancer presented with a hemoglobin (Hgb) of 5.1 g/dL and a platelet count of 39K/uL. His cytopenias had progressively worsened over the past 6 months from a baseline Hgb of 12 gm/dL and platelet count of 222 K/uL, and were secondary to bone metastases and prior spinal radiation. Thirty days prior to presentation, he received one unit of PRBCs at an outside institution for a Hgb of 7.0 g/dL. Due to worsening Hgb and clinical symptoms, a direct antiglobulin test (DAT) was obtained 21 days after the transfusion. It was noted to be positive for C3d suggesting a delayed hemolytic transfusion reaction. Upon type and cross match he was found to be O+ with new antibodies to Jka and U. A single compatible unit of PRBCs was identified and transfused. His Hgb increased from 4.5 g/dL to 5.9 g/dL with transfusion. His Hgb remained near 5g/dL over the next week however, he remained very symptomatic. There were no additional matched PRBCS available for transfusion despite a nationwide search. Given the dire clinical circumstances, it was decided to administer the commercially available C1-INH, Berinert®, prior to transfusion of antigen mismatched Jka-, U+ PRBCs. He received C1-INH 2500 units (roughly 20u/kg) prior to the first unit of Jka-, U+ PRBCs without clinical complication and improvement of Hgb from 5.4 g/dL to 7.1 g/dL. Twenty four hours later he received a second dose of C1-INH 2500 units followed by an additional unit of Jka-, U+ PRBCs. There were no clinical signs of a HTR, and the Hgb improved to 8.6g/dL. He received 5 additional doses of C1-INH in tapering doses in an attempt to prevent delayed HTR. The Hgb remained above 6 g/dL for the remainder of his hospital stay. The LDH initially increased from 642 units/L to 936 units/L 72 hours after the second premedicated mismatched transfusion. The haptoglobin level remained < 8 mg/dL until 5 days post the second mismatched transfusion after which it returned to the normal range. The improvement in markers of hemolysis and stabilization of Hgb suggests that any further hemolysis related to the transfusion of the 2 Jka - U+ units was minimal and clinically insignificant. Quantification of anti U antibodies did not demonstrate an increase when evaluated 16 days post transfusion of U+ PRBCs.
We present the first reported case where antigen mismatched PRBCs were safely transfused in conjunction with prophylactic complement inhibition with C1 esterase inhibitor. The transfusions were well tolerated, lacking clinical signs of hemolysis, and resulting in an appreciable and sustained improvement in Hgb. The additional observation of a stable anti U antibody titer 2 weeks after the transfusion of U+ PRBCs to U- patient is thought-provoking. Future directions would include clinical trials exploring complement inhibition's role in preventing HTRs and preventing alloantibody formation in heavily transfused patients.
Broome:True North Therapeutics: Honoraria; Alexion Pharmaceuricals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal