Abstract
Chronic transfusion therapy (CTT) for sickle cell anemia (SCA) reduces the risk of sickle complications by diluting hemoglobin S (HbS)-containing red blood cells (RBCs) with HbA RBCs and suppressing sickle erythropoiesis. HbS level is influenced by both endogenous hemolysis and clearance of transfused RBC. Minor RBC antigen (Ag) mismatches may result in alloimmunization and hemolytic reactions, but it is unknown if Ag mismatches independently influence HbA clearance. This study sought to: (1) determine the frequency of RBC minor Ag mismatches that occur in chronically transfused SCA patients receiving phenotype-matched transfusions; (2) determine the rate of clearance of transfused HbA following each transfusion; (3) explore associations of HbA clearance with RBC minor Ag mismatches.
Children with SCA ages 3-20 years on CTT by either simple transfusions or partial manual exchange (PME) were enrolled in a prospective observational study. All RBC units were serologically matched for C/c, E/e, K (category 1); additional matching for Fya, Jkb, and Ag-negative for putative antibodies (category 2) was provided to patients with ≥1 clinically significant alloantibody or (in some cases) warm autoantibodies. RBC genotyping of SCA patients was performed using PreciseType™ Human Erythrocyte Antigen (HEA), RHCE, and RHD BeadChip assays (Immucor, Norcross, GA). Genomic DNA was extracted from unit segments of all RBC transfused over 12 months and tested using the prototype HEA Leukoreduced (HEA-LR) BeadChip assay to detect the same profile of RBC Ag. RH variant testing of units was not performed.
Pre-transfusion HbA was recorded for all episodes, where HbA (g/dL) = Hb (g/dL) x HbA%. Hb electrophoresis was drawn 15-30 minutes post-transfusion in a subset of episodes. For other episodes, post-transfusion HbA was estimated based on the volume of transfusion (VolT), estimated unit hematocrit (Hct), phlebotomy volume (VolPh, if applicable) and total blood volume (TBV), using the following equation:
Post HbA (g/dL) = Pre HbA (g/dL) + [(unit Hct) x VolT / 3 x Wt] - (Pre HbA%/100) x [(Pre Hb (g/dL) x VolPh / 3 x TBV].
HbA loss/day was calculated from the Post HbA to the next pre-transfusion HbA.
There were 82 patients (54 category 1, 28 category 2) who received 2123 units in 1014 transfusion episodes; HEA-LR genotypes were obtained on 1827 units and 828 complete transfusion episodes. Altered RHC/c or e genotypes were present in 29 (36%), and partial D+ genotypes in 4 (5%). There were 46 historical alloantibodies, the majority against Rh (C, E, hrB, Goa, f, V, VS) and Kell (K, Kpa, Jsa). During the study, 5 patients developed 6 new alloantibodies: 3 Jsa, 1 Goa, 1 Wra, 1 AUS (category 2 transfusions alloantibody incidence 0.64/100 units; category 1 transfusions 0.074/100 units). The mean genotype-predicted Ag mismatches per transfusion was 3.52 for category 1 and 2.78 for category 2 (despite category 2 being matched for ≥2 Ags beyond category 1 matching). Ag mismatch frequency was different in category 1 vs. 2 transfusions for: C (3.8% vs. 0%, p=0.0009), e (24.5% vs. 36.8%, p=0.0002), and VS (21.2% vs. 13.6%, p=0.008). Overall concordance between HEA-LR and available serologic testing was 98%.
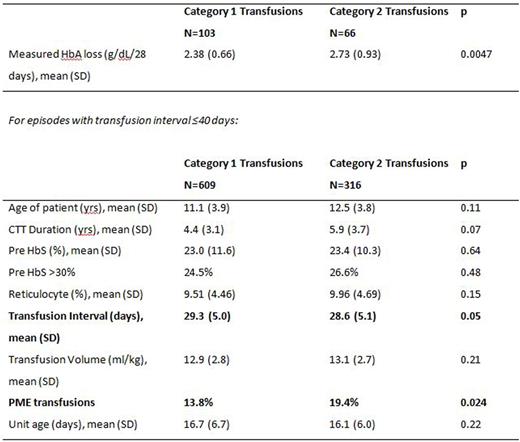
Of the 169 transfusion episodes with post-transfusion HbA measurements (103 category 1, 66 category 2), the mean HbA loss was 2.38 g/dL/28 days for category 1 vs. 2.73 g/dL/28 days for category 2 (p=0.0047). Comparison of category 1 vs. 2 transfusions (see table) shows shorter transfusion interval and higher frequency of PME in category 2 transfusions. Estimated post HbA had a bias of -0.31 g/dL (95% C.I.-0.18, -0.46) and precision of 0.85 g/dL (95% C.I. 0.61, 1.18). Comparison of estimated HbA loss to Ag mismatches was limited to 533 transfusion episodes in which unit Hct could be estimated within <10% range. Estimated HbA loss had a small negative correlation with Ag mismatches for category 1 (r= -0.123, p=0.027) but not for category 2 transfusions. In linear regression, the only Ag associated with increased HbA loss was N which was limited to category 1 transfusions (p=0.027). HbA loss did not correlate with age of units.
This is the first report to characterize RBC minor Ag mismatches in SCA patients on CTT receiving limited vs. extended phenotypically matched RBCs. These findings suggest that clearance of transfused RBCs may be influenced by the patient's history of immunologic response to RBC Ag rather than total or specific Ag mismatches.
Josephson:Octapharma: Consultancy; Immucor: Consultancy; Biomet Zimmer: Consultancy. Winkler:Cerus Corporation: Honoraria; Siemens Healthcare Diagnostics, Inc.: Honoraria; Instrumentation Laboratory: Employment, Honoraria, Membership on an entity's Board of Directors or advisory committees; Grifols: Membership on an entity's Board of Directors or advisory committees; HemoCue America: Membership on an entity's Board of Directors or advisory committees. Fasano:Terumo BCT: Other: educational speaking (non-branded) on Iron overload in SCD; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: educational speaking (non-branded) on Iron overload in SCD; Apopharma: Membership on an entity's Board of Directors or advisory committees; Immucor: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal