Abstract
Background: Venous thromboembolism (VTE) is an emerging hospital acquired complication in pediatric hospitals. Substantial efforts have been made to identify high-risk population based on exposure to risk-factors for VTE. Our group developed the PedsClot Clinical Decision Rule (Sharathkumar et al Journal of Thrombosis and Haemostasis, 10: 1326-1334) to identify this high-risk population based on a case-control study but this has not been validated yet.
Objective: To validate thePedsClot Clinical Decision Rule [PDCR] through prospective collection of data in a tertiary pediatric hospital. This study reports our findings of interim analyses for process evaluation.
Methods: This prospective data collection was performed using the Lurie Children's hospital automatic data import facility through the Enterprise Data Warehouse[study period: 02/01/2012-12/31/14]. Real time data was added to a research database of consecutive admissions based on following inclusion criteria: > 48 hour stay in the Intensive Care (Pediatric, Cardiac, Neonatal), Hematology Oncology and Infectious Disease units. Following variables were included in the dataset: age, sex, ethnicity, date of admission and discharge, ICU admission, central venous catheter (CVC), blood stream infection, immobilization, oral contraceptives, mechanical ventilation> 12hrs, and length of stay. Risk factors were weighed and scored as per PDCR rule. Chi-square tests were used to examine the association between the potential risk factors and VTE. PDCR model performance was evaluated by reporting the sensitivity and specificity.
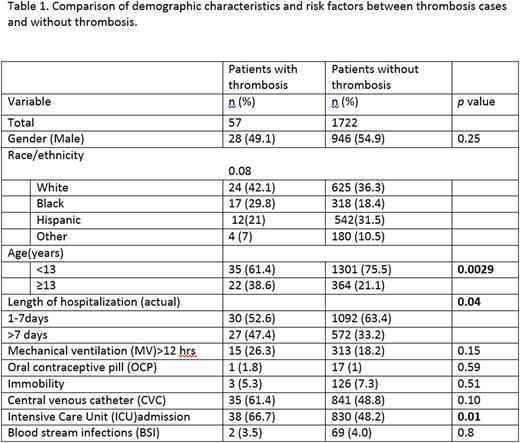
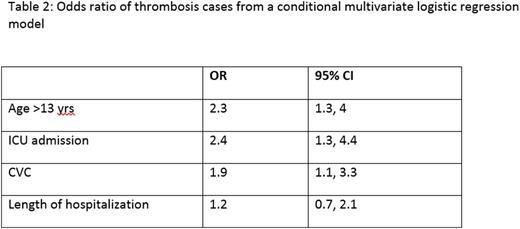
Results: A total of 1722 children were eligible for the interim analyses. The demographic and clinical features of the dataset analyses and the odds ratio [OR] are detailed in Table 1 and 2 respectively. Of 1722 patients, 57 (3.3%) were identified as VTE, 50% were admitted to the ICU and 51% had a CVC. VTE was associated with age ≥ 13 years (AOR=2.3; 95% CI=1.3, 4.0), ICU admission (AOR=2.4; 95% CI=1.3, 4.4), and CVC (AOR=1.9; 95% CI=1.1, 3.3). The model performance showed that at the cut off point of 3, the specificity of the PDCR in predicting VTE was 75% but had a low sensitivity (37%). Risk prediction variables such as assessment of immobilization, use of oral contraceptives and prediction of hospital stay required manual entry into the datasheet; this makes data-capture labor intensive, especially for larger datasets.
Conclusions: This study utilized technological advances and database warehouse facility to validate PDCR. This interim data analyses indicates that in real life, PDCR has a high specificity but poor sensitivity in identifying children with predisposition for VTE. We are currently working to refine the risk model which may lead to a better model performance.
Sharathkumar:Bayer, Baxter, CSL Behring: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal