Abstract
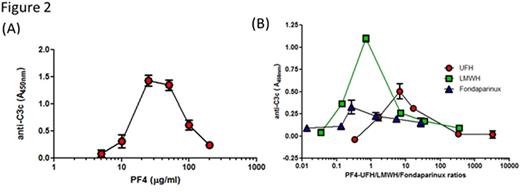
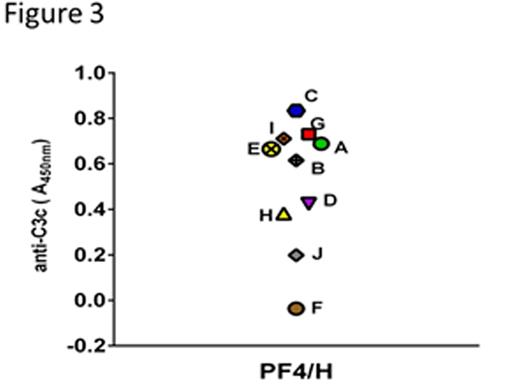
The immune response to platelet factor 4 (PF4)/heparin complexes is a frequent iatrogenic complication of heparin therapy associated with development of heparin-induced thrombocytopenia (HIT). Our recent studies indicate that PF4/heparin complexes potently activate complement (C') in healthy donors and patients receiving heparin therapy. In these studies, we also show that C' mediates selective antigen-binding to circulating B cells via the complement receptor 2, CD21 (Khandelwal, Blood 2016). In the course of performing these studies, we developed a simple enzyme-linked immunosorbent assay (ELISA)-based technique for measuring C3 subunit generation by protein/heparin complexes in plasma. For this assay, monoclonal antibodies to PF4/heparin (KKO; Arepally, Blood 2000) or protamine (PRT)/heparin complexes (ADA, Lee unpublished data) are incubated overnight on a microtiter plate, followed by washing and blocking with 1% bovine serum albumin (BSA) in phosphate buffered saline (PBS) for 2 hours. To examine C' activation, plasma is incubated with buffer or antigen (PF4, 25µg/mL ± heparin or PRT 31 µg/mL ± 4U/ml heparin) for 60 minutes at 37°C followed by addition of 10mM EDTA to inhibit further C'activation. Plasma containing antigen and activated C' fragments is next added to the antibody coated plate for 1 hour at 40C followed by three washes. C' activation is detected using a biotinylated antibody to C3c (Quidel Corporation, San Diego, CA) followed by streptavidin-HRP (BD Bio Sciences San Jose, CA). Using this assay, we show that plasma incubated with PF4/heparin complexes, but not PF4 alone or heparin alone trigger C' activation as measured by C3 binding (1:50 plasma dilution shown; Figure 1A). Similarly, using ADA, a recently developed monoclonal antibody to PRT/heparin complexes, we show that PRT/heparin complexes, but not PRT alone or heparin alone, robustly activate C' (1:10 plasma dilution shown; Figure 1B). To show that C3 generation is dependent on ultra-large complex (ULC) formation, we performed experiments using a fixed dose of UFH (0. 5 U/mL) and varying doses of PF4 (5-200 µg/mL) or fixed dose of PF4 (25 µg/mL) and varying doses of UFH (0.0005-5.0 U/mL), LMWH (0.01-100 µg/mL) and fondaparinux (0.05-100µg/mL). Consistent with published observations (Khandelwal, Blood 2016), we note that changes in PF4 concentration (Figure 2A) or UFH/LMWH/fondaparinux concentration (Figure 2B) results in a bell-shaped curve of C'activation that mirrors ULC formation. Because the immune response to PF4/heparin is highly variable among heparin-exposed patients, we examined inter-individual variation in C' activation by PF4/heparin complexes. For these studies, we analyzed C' activation using a fixed dose of PF4/heparin (25 µg/mL PF4 and 0.25U/mL heparin) in freshly collected plasma from 10 healthy donors . As shown in Figure 3, we noted that C' activation is highly variable among donors, with some donors showing significant C' activation (donors A,B,C, E ,G,I), while others show minimal C3 generation (donors D,F,H,J) in response to same dose of PF4/heparin complexes. Together, these studies show that the ELISA-based C3 generation assay is a simple, robust assay for detecting C' activation by PF4/heparin or PRT/heparin complexes and can be useful in studying mechanisms of C' activation and biologic effects of commercial heparins.
Arepally:Biokit: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal