Abstract
Background: Atypical hemolytic uremic syndrome (aHUS) is a rare disease predominantly caused by chronic, uncontrolled complement activation, leading to systemic thrombotic microangiopathy (TMA). The aHUS Registry is a noninterventional global initiative established in April 2012 (NCT01522183). Overall objectives of the Registry are to collect information on patient outcomes, regardless of treatment approach, as well as safety and effectiveness data specific to the use of eculizumab, a terminal complement inhibitor approved for treatment of aHUS. The objective of the current analysis was to characterize organ involvement in patients with aHUS, who were enrolled in the global aHUS Registry, prior to eculizumab treatment.
Methods: Eligible patients have clinical diagnoses of aHUS, regardless of whether a complement abnormality had been identified or how the disease was managed. Demographic and medical/disease history data are collected retrospectively prior to enrollment and prospectively following enrollment. All treatment modalities are captured, including eculizumab.
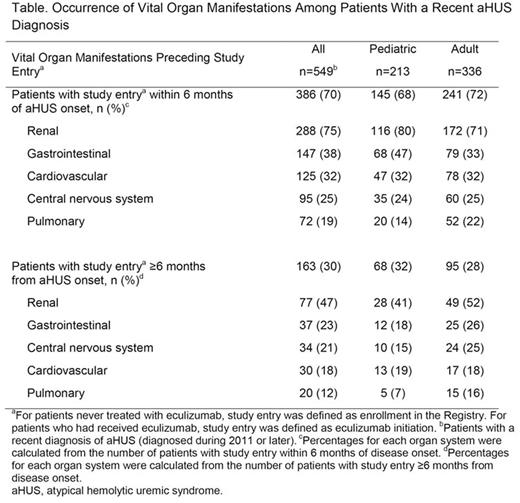
Results: This analysis included 851 patients; 387 (46%) pediatric and 464 (55%) adult onset patients. Study entry within 6 months of aHUS onset was reported by 39% of pediatric and 52% of adult patients. In the 6 months prior to study entry, renal manifestations were reported in 34%, gastrointestinal in 18%, cardiovascular in 15%, central nervous system in 11%, and pulmonary in 9%. Overall, extrarenal manifestations occurred more frequently in adult patients compared with pediatric patients. Due to the mainly retrospective data collection and to avoid recollection bias, a subgroup of recently diagnosed (ie, during 2011 or later) patients were specifically analyzed (n=549). While systemic manifestations were reported among patients after initial presentation (time from aHUS onset to study entry ≥6 months) in 12% to 47% of patients (Table), they were more frequent among patients with study entry within the first 6 months of disease onset (19% to 75% of patients). Overall, the most frequent organ manifestations occurring among patients with study entry within 6 months of disease onset were renal (75%), gastrointestinal (38%), cardiovascular (32%), central nervous system (25%), and pulmonary (19%). Pediatric patients experienced more gastrointestinal and fewer pulmonary manifestations than adult patients.
Conclusions: In this large cohort of patients with aHUS, extrarenal manifestations were frequently reported at the time of disease onset. In light of the differential diagnosis of TMA, this highlights the importance to consider non-renal manifestations as a possible consequence of aHUS. Of interest, systemic involvement was still common ≥6 months after disease onset, emphasizing the importance of continued evaluation of organs in addition to the kidney.
Scully:Alexion Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding. Johnson:Alexion Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees. Kupelian:Alexion Pharmaceuticals, Inc.: Employment. Greenbaum:Alexion Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal