Abstract
Introduction: Acute ischemic stroke (AIS) is a hypoxic ischemic disorder associated with a sterile inflammatory reaction. Neuronal injury from ischemic stroke is aggravated by invading peripheral polymorphonuclear cells (PMNs). Neutrophils not only have a remarkable neurotoxic effect from the release of proteolytic enzymes, but also foster coagulation cascade by exposing membrane phosphatidylserine (PS), aggregating to platelets and releasing neutrophil extracellular traps (NETs). However, the role of circulating neutrophils in hypercoagulation state after AIS remains unclear. Our aims were to determine the procoagulant role of circulating neutrophils after AIS, and to elucidate the mechanism of neutrophils-induced thrombophilia.
Methods: 73 newly diagnosed AIS patients and 35 risk factors matched controls were enrolled. Patient blood samples were collected at 6 h, 12 h, 24 h, 3 d, and 7 d after the onset of clinical symptoms. PS exposure on neutrophils and neutrophil-platelet aggregation was measured by flow cytometry and confocal microscopy. The percentage of NETs-releasing PMNs was quantified by confocal microscopy. Double-stranded DNA and myeloperoxidase-DNA complexes were also measured as in vivo markers of NETosis. Specifically, dsDNA was quantified using the Quant-iT PicoGreen dsDNA Assay Kit. Myeloperoxidase-DNA complex was measured using a capture ELISA.The procoagulant activity (PCA) of neutrophils was measured by clotting time and purified coagulation complex assays. Plasma levels of coagulation activation were evaluated by thrombin-antithrombin experiment.
Results: The initial levels of PS+ neutrophils and neutrophil-platelet aggregation were 2.1- and 1.8-fold higher, respectively, in AIS than controls. Specifically, PS+ neutrophils peaked at 24 hours and returned to their initial levels at 3 days while neutrophil-platelet aggregation elevated at initial and remained high for the later time points. Patient neutrophils supported significantly shortened clotting times and increases in Xase activity and thrombin formation compared to controls (P < 0.001 for all). Interestingly, treatment with lactadherin, a PS antagonist, effectively restored patient PCA to control levels. The amounts of NETs+ cells, dsDNA and myeloperoxidase-DNA complexes were elevated at 3 days after stroke and positively correlated with thrombin generation. Furthermore, cultured endothelial cells were intensely activated by neutrophils of stroke patients and subsequently supported massive fibrin formation. Moreover, blockade of NET formation with DNAse I inhibited fibrin formation by approximately 60%. In a subanalysis, 27 patients with early infections were matched with 27 patients without infections according to S100B peak levels. These two patient subgroups showed significant differences in the temporal pattern of PS+ neutrophils, neutrophil-platelet aggregation, NETs%, markers of NET formation, and coagulation activation. The levels of coagulation markers were significantly higher in patients with early infections than in patients without (P < 0.05 for all).
Conclusions: The thrombophilic susceptibility could be partly due to the activation of neutrophils after ischemic stroke. AIS patients with early infections are more prone to thrombosis. Our studies identify PS exposure on neutrophils and formation of NET as potentially novel therapeutic targets in the treatment of AIS.
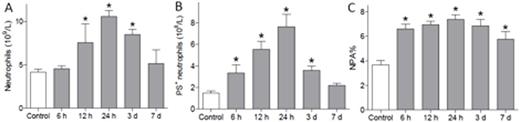
The changes in the number of neutrophils, PS+ neutrophils, and neutrophil-platelet aggregation (NPA) measured within 7 days of stroke. *P < 0.05 vs. control.
The changes in the number of neutrophils, PS+ neutrophils, and neutrophil-platelet aggregation (NPA) measured within 7 days of stroke. *P < 0.05 vs. control.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal