Abstract
Introduction: White matter strokes are a major concern for patients with sickle cell disease and other forms of anemia. SCD patients are known to have diffuse white matter volume loss, abnormal WM microstructure, silent strokes, and poor neurocognitive function. Similar neurological abnormalities have been described in thalassemia intermedia. Low levels of hemoglobin have been associated with risk of silent cerebral infarctions across patient populations with chronic anemia and severe anemia may increase the risk for cerebral vascular accidents. In this current study, we use MRI brain mapping techniques to explore the relationship between a wide range of hemoglobin levels and WM neural tissue properties.
Methods: 16 SCD patients (age=26.1 ± 10.3; F=9, M=7; hemoglobin=9.4 ± 2.1), 8 patients with non-sickle anemia (age=23.6 ± 7.4; F=4, M=4; hemoglobin=12.2 ± 3.2), and 15 control subjects (age=22.8 ± 7.2; F=10, M=5; hemoglobin=13.2 ± 1.3) were recruited with informed consent or assent; the study was approved by the Institutional Review Board at Children's Hospital Los Angeles (CCI#11-00083). Exclusion criteria included pregnancy, previous overt stroke, acute chest, or pain crisis hospitalization within one month. MRI data were acquired on a 3T Philips Achieva (v.3.2.1) using an 8-channel head coil.
3D T1-weighted images (TE =3.8ms TR =8.3ms; resolution = 1mm3) were pre-processed using BrainSuite (brainsuite.org) and registered to a common atlas space using Surface Volume Registration (SVReg15a). Diffusion weighted images (TE = 2.5ms; TR = 4.8ms; 2.5mm isotropic voxels) included 30 diffusion-encoding directions at b-value=1000m/s2 and a reverse-gradient b=0. Fieldmaps were estimated using FSL's topup module. BrainSuite Diffusion Pipeline (BDP15a) performed distortion correction, co-registration to T1-weighted images, and diffusion tensor modeling. Voxel-wise calculations of DTI metrics including fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD) were transferred to the common atlas space. Pearson correlations were performed using BrainSuite Statistics Toolbox.
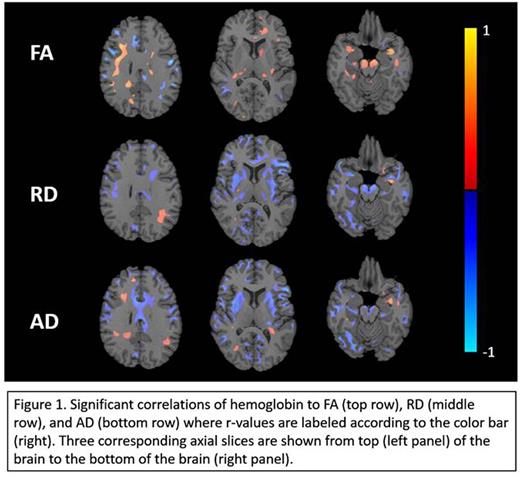
Results: Hemoglobin was correlated to FA, AD, and, RD in multiple brain regions after controlling for age and sex (Figure 1) The colorbar represents the r-value for the correlations (red positive, blue negative) and only statistically significant values are displayed. Anemia was associated with increased RD and AD broadly over the brain (blue), including corpus callosum, subcortical nuclei, and frontal and parietal white matter. These regions represent classic "watershed" areas that are the primary targets of silent strokes in sickle cell disease, hypertension, aging, and obstructive sleep apnea. FA changes were more modest in extent, but also occurred in areas classically associated with silent stroke.
Conclusion: Our study suggests that the severity of anemia, rather than the type of anemia, is the primary risk factor for white matter damage. Low hemoglobin concentration correlated to increases in RD and AD, indicative of demyelination and axonal degeneration respectively. Interestingly, the regions of abnormal diffusivity parallel the pattern of regional vulnerability to stroke in SCD patients in previous literature (frontal lobe > parietal lobe, subcortical nuclei, > temporal lobe, >> occipital lobe and cerebellum). While silent strokes and abnormal diffusivity co-localize, the volume of infarcted tissue is much smaller, suggesting that changes in RD and AD are detecting preclinical white matter injury in anemic patients. We postulate that anemia, by depleting cerebrovascular reserve, leaves patients vulnerable to acute interruptions in supply or increases in metabolic demand. Our observations may have important implications for choosing set points for chronic transfusion therapy in anemic patients.
Wood:Biomed Informatics: Consultancy; Biomed Informatics: Consultancy; Apopharma: Consultancy; Apopharma: Consultancy; AMAG: Consultancy; AMAG: Consultancy; Celgene: Consultancy; Celgene: Consultancy; World Care Clinical: Consultancy; World Care Clinical: Consultancy; Vifor: Consultancy; Vifor: Consultancy; Ionis Pharmaceuticals: Consultancy; Ionis Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal