Abstract
Introduction: Oral iron delivery is attractive due to the ease of administration and is mainly based on immediate release formulations of ferrous iron. Commonly used ferrous iron salts are poorly absorbed. Overcoming gastrointestinal barriers, is a great challenge and there is a need for new absorption and protective enhancers. Iron DeficiencyAnemia (IDA) is a presenting symptom in approximately 40% of patients with Hodgkin's Lymphoma (HL). Usually with hemoglobin (Hb) between 10 and 12 g/dL. When considering oral iron therapy in cancer patients one must take into account the impact of worsening of existing clinical conditions due to oral iron as intolerance and non-compliance. For these reasons, effective management of iron deficiency anemia is mandatory and iron supplementation is often necessary. To avoid constant exposure of intestinal cells to insoluble iron and consequent side effects, we have developed a new oral Iron formulation named Sucrosomial¨ Iron. This oral formulation is an innovative preparation of ferric pyrophosphate, covered by a phospholipids plus sucrester matrix, with high bioavailability and tolerability and has been showed to improve Hb concentration similarly to intravenous iron but without any gastrointestinal side effects.
Objective: to showpre-clinical and clinical evidences of the absorption, effectiveness and tolerability of Sucrosomial¨ Iron.
Methods: to study the absorption pathway of Sucrosomial¨ Iron, gastro-resistance and permeation experiments have been performed using in vitro simulated gastric pH digestion, and isolate rat intestine ex vivo models, respectively. To show the efficacy and compliance of Sucrosomial¨ Iron (Sideral¨ Forte) in IDA patients with Hodgkin's Lymphoma (HL), 25 patients were retrospective analyzed, (median age, 35 years; range 26 to 44 years). All patients were staged 2B or higher, according to Ann Arbor classification, none of them showed bone marrow infiltration. A continued treatment with oral Sideral¨ Forte (30 mg/die) was performed for the whole period of chemotherapy administration and iron parameters were tested at the end of the planned treatment.
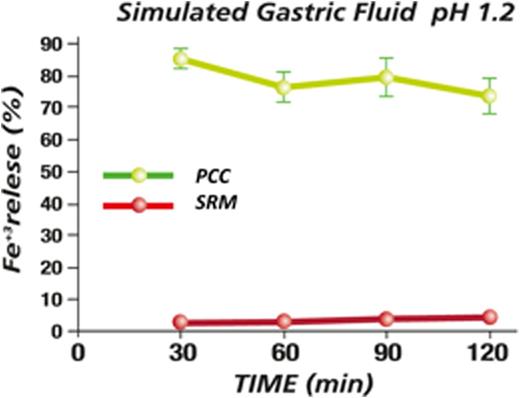
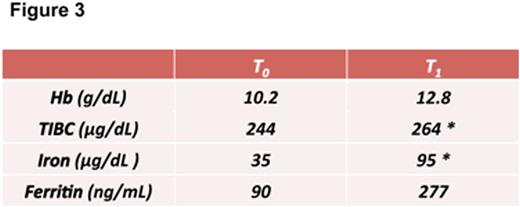
Results: In vitro gastro-resistance experiment displays the ability of Sucrosomial¨ Iron (SRM), to avoid release of Ferric Iron from the matrix compared to phospholipid only-containing iron formulation (PCC, Figure 1). These results showed the gastro-resistance features of SRM.We have performed ex-vivo permeability experiments using Hussing chamber-like experimental model and measured ferric iron concentration in presence of bivalent iron chelator (BPDS) too. Thanks to this model we have showed that SRM is able to cross the intestinal tissue through a passive transport in respect to the other iron formulations (Figure 2). Moreover we have observed that Sucrosome components ratio is pivotal for kinetics properties of Sucrosomial¨ Iron. Data from the clinical study showed that Sideral¨ Forte is well tolerated and effective in patients with HL in advanced stage. At baseline (T0) medium Hb level was 10.2 g/dL, median serum iron and total iron-binding capacity (TIBC) were 35 μg/dL and 244 μg/dL respectively. Ferritin levels showed a wide variation with a median of 90 ng/mL. At the end of treatment (T1 6 months) medium Hb level was 12.8 g/dL (+2,2 g/dL) the median serum iron and total iron-binding capacity increased up to 95 μg/dL and 264 μg/dL and Ferritn levels increased with a median of 277 ng/mL (Figure 3). Thanks to the high compliance and tolerability, all patients were able to continue Sucrosomial¨ Iron supplementation up to the end of the chemioterapy period.
CONCLUSIONS: these results showed that Sucrosomial technology is able to protect ferric iron from the gastric pH conferring the ability to reach covered the intestine for absorption and to cross intestinal epithelium through a passive route; thus minimizing the conventional oral iron side effects and increasing Hb and Ferritin levels in HL patients.
SRM Iron pyrophosphate, Lecithin, Sucrester
PCC phospholipid-containing carrier
BPDS Bivalent Iron chelator
PYR Iron pyrophosphate
SLP pyr, Lecithin, low phospholipid concentration, Sucrester
SRMS pyr, Lecithin, high Sucrester concentration
Results are expressed as median values
*p<0,05
Brilli:Pharmanutra S.p.A.: Employment. Tarantino:Pharmanutra S.p.A.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal