Abstract
Background: There is compelling evidence that physical activity positively influences quality of life (QoL), and health-related outcomes including improved muscle mass and physical functioning in cancer pts. SCT pts however have unique barriers to exercise including isolation, restriction of activities, and treatment toxicity. In the early post SCT period, pts describe worsening fatigue, physical capacity and QoL. We sought to determine whether a partially supervised exercise intervention early post SCT would address these issues. Our primary objective was to determine the feasibility of delivering such an intervention at our institution. Secondary objectives were to assess changes as a result of the intervention in QoL, muscle mass and physical functioning.
Methods: From Aug 2015-Jun 2016, we conducted a prospective single-arm study to evaluate feasibility of a 12 week partially supervised exercise program (1 supervised, 2 unsupervised sessions/week) for alloSCT pts with hematologic malignancies. The program consisted of 3 progressive endurance (stationary bike, walking) and 2 resistance training sessions/week, from hospital discharge (D/C) to Day (D) 100. Feasibility was defined as ability to recruit >65% of eligible pts, ≥70% retention and ≥70% adherence. Secondary outcomes were measured pre SCT (T0), at D/C (T1) and D100 (T2) and included QoL, muscle strength, mobility, aerobic fitness and body composition. Changes from T0 to T1 and T1 to T2 were compared using a paired sample t-test.
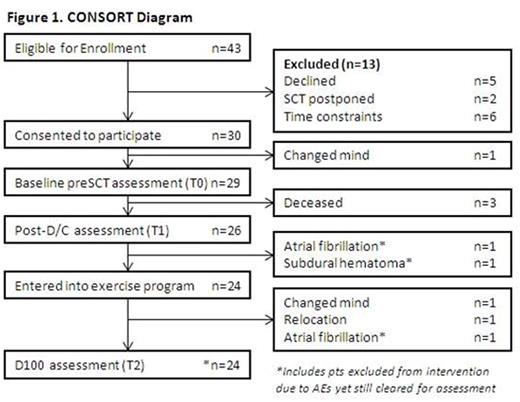
Results: Of 43 consecutive alloSCT pts assessed for eligibility, 30 (70%) entered the study: 17 male (57%), median age 48 yrs (range 19-66 yrs). Transplants characteristics were: related 6; unrelated 24; myeloablative 23; reduced intensity 7. At baseline, SCT comorbidity index was 0 in 43%, 1-2 37%, ≥ 3 20%. Pts self-reported exercising enough to break a sweat (Godin et al) never 60%, sometimes 33%, often 7%. Median hospitalization was 29d (range 15-141); 9 pts developed grade II-IV acute graft-versus-host disease (skin 7, gut 5, liver 1).
Overall retention to D100 was 80% (Figure 1). Two pts had complications during hospitalization and did not enter the program. Of the 24 pts who entered the program, adherence was 72% for supervised and 89% for unsupervised sessions. Logistics with scheduling around multiple medical appointments in the early post SCT period were the most common reasons for non-adherence. Other reasons included nausea, fatigue and weakness. One pt developed exercise-induced atrial fibrillation in week 3 requiring cessation of exercise.
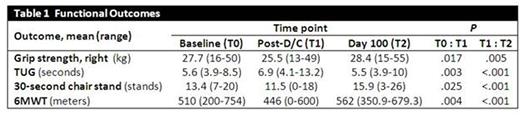
When comparing secondary outcomes from T0 to T1 (Table 1), pts had significantly decreased muscle strength (grip strength, 30-second chair stand), mobility (timed up-and-go [TUG]) and aerobic capacity (6-min walk test [6MWT]). At T2 after completion of the program, these measures all increased significantly when compared to T1. Global QoL scores on the EORTC measure decreased from T0 to T1 (P=.011) and were significantly improved by T2 (P<.001). Similar trends were seen across functional and symptom measure scales. Body composition comparisons between T0 and T2 demonstrated a trend towards increased appendicular lean mass (P<.059).
Conclusion: Results of this pilot study demonstrate feasibility of a partially supervised exercise program post-alloSCT deemed by achieving the target recruitment rate, ≥70% adherence and ≥70% retention. Logistics were the most common reason for non-adherence, highlighting need for a multidisciplinary team with knowledge of the post SCT setting. Prior observational studies have shown significant declines in physical functioning and QoL within the first 100 days of SCT. In contrast we demonstrate significant improvements in these measures. Furthermore, we demonstrate a trend towards increased lean muscle mass, which is a novel secondary outcome that warrants further evaluation in this setting. Our findings support the need for this clinical intervention and will be evaluated in a larger randomized trial.
Gerrie:Roche Canada: Research Funding. Plantinga:BCCA: Employment. Broady:Lotte & John Hecht Memorial Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal