Abstract
Introduction: Pre-treatment characteristics of pts with CLL, such as del(17p), are used to identify high-risk populations for enrollment onto clinical trials. Nevertheless, little is known about how to identify high-risk pts who are not captured by conventional prognostic tools, yet may benefit from clinical trials. Studies have shown that first-line chemoimmunotherapy (CIT) provides a therapeutic challenge to CLL and confers a selective pressure favorable to the growth of aggressive clones (Landau et al. Nature 2015;526:525-30). Early progression of disease (POD) may reflect specific aspects of disease biology and serve as a post-treatment prognostic marker that defines high-risk pts. Here we describe the characteristics and survival of pts experiencing early POD within 2 years of treatment initiation.
Methods: The Connect CLL Registry (NCT01081015), a multicenter, prospective observational cohort study, enrolled 1,494 CLL pts between 2010-2014 from 179 community, 17 academic, and 3 government sites in the USA. Pts were ≥ 18 years and were enrolled ≤ 2 months of initiating any line of therapy (LOT). Of 889 pts who received first LOT (LOT1), 154 were excluded due to subsequent treatment or death without recorded progression. Pts were stratified by early POD (progression with or without transformation ≤ 2 years of treatment initiation), or late/no POD. A 2-year cutoff was found to approximate median time to POD. Cox regression models were used to identify independent predictors of progression or death. Progression-free survival probabilities were estimated by the Kaplan-Meier method and compared between subgroups using the log-rank test.
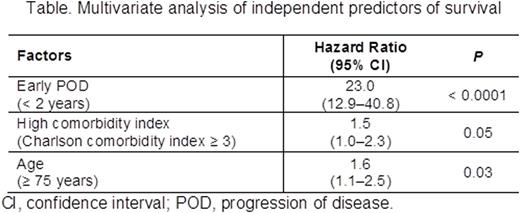
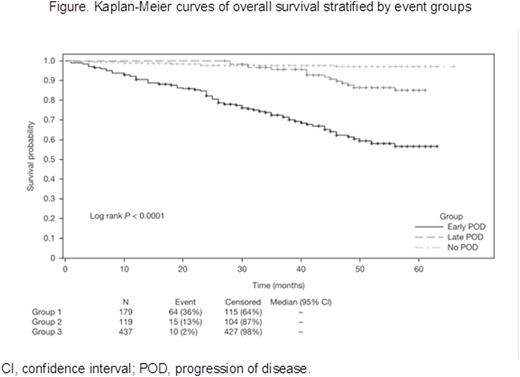
Results: A total of 735 pts with CLL were included in the analysis. Median age was 67 years (range 22-99), 64% were male, 29% had advanced Rai stage (III/IV), and 14% of those with available FISH data had del(17p). At a median follow-up of 42.5 months, 298 (40.5%) pts had POD. Median time to POD was 20 months. A total of 179 (24.3%) pts experienced early POD (≤ 2 years); the remaining 556 pts experienced late POD (> 2 years; 119 [16.2%]) or no POD (437 [59.4%]). Early and late/no POD subgroups showed similar distribution of sex, race, Rai stage, and Charlson comorbidity index (all P > 0.05). The early POD group had a higher median age (70 vs 67 years; P = 0.025), and more frequently had del(17p) (20 [20%] vs 22 [7%], among pts with available FISH; P = 0.001) than the late/no POD group. Comparison of first-line treatment patterns showed pts with early POD were more likely to receive monotherapy and less likely to receive combination CIT compared to pts in the late/no POD group. The most commonly used monotherapy was rituximab (15.1% in early POD vs 11.8% in late POD vs 9.2% in no POD), followed by chlorambucil, bendamustine, fludarabine, and cyclophosphamide. Overall response rates were lower in the early POD group (54% vs 83%; P < 0.001). The 2 subgroups had similar rates of combination CIT and monotherapy use as subsequent treatment following POD. Of 11 pre-, intra- and post-treatment factors associated with survival in univariate analysis (P < 0.1), 3 were independent predictors in a multivariable analysis: early POD, age ≥ 75 years, and high comorbidity index. Notably, treatment duration ≤ 4 months and del(17p) were associated with survival in univariate models but were not independent predictors in a multivariable model. Multivariable analysis revealed pts experiencing early POD had a significantly higher risk of death than those who did not experience early POD (HR 23.0, 95% CI 12.9-40.8; P < 0.0001; Table). Three-year survival was 72% vs 97% (P < 0.0001; Figure) for early POD vs late/no POD. To exclude initial therapy as a possible confounding factor for survival, analyses were repeated on those pts in LOT1 receiving fludarabine, cyclophosphamide, and rituximab (FCR, n = 197) and bendamustine plus rituximab (BR, n = 161), the most common CIT regimens. For pts receiving FCR, the early POD group had inferior survival (HR 47.5, 95% CI 12.3-183.6; P < 0.0001). Similar results were observed in pts treated with frontline BR (HR 60.5, 95% CI 13.2-277.6; P < 0.0001).
Conclusion: Disease progression ≤ 2 years after initiation of frontline therapy is an independent negative predictor of survival in pts with CLL. This trend is observed across different treatment regimens. This clinical risk factor identifies a subgroup of high-risk pts who should be considered for enrollment onto clinical trials.
Farber:Seattle Genetics: Research Funding. Davids:Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie, Janssen, Gilead: Consultancy; Celgene Corporation: Consultancy. Grinblatt:Celgene Corporation: Consultancy, Speakers Bureau. Lamanna:Gilead: Research Funding; AbbVie: Research Funding; Genentech: Research Funding; Infinity: Research Funding; Pronai: Research Funding; Celgene Corporation: Research Funding. Mato:Abbvie, Acerta Pharma, Gilead Sciences, ProNAi, TG Therapeutics, Theradex: Research Funding; Abbvie, Gilead Sciences, Pharmacyclics, TG Therapeutics: Consultancy. Nabhan:Celgene, Genentech, Abbvie, Infinity, Cardinal Health: Consultancy; Celgene, Genentech, Seattle Genetics, Astellas: Research Funding. Kiselev:Celgene: Employment, Equity Ownership. Swern:Celgene: Employment, Equity Ownership. Flick:Celgene Corporation: Employment, Equity Ownership. Bhushan:Celgene Corporation: Employment, Equity Ownership. Sullivan:Celgene Corporation: Employment, Equity Ownership. Sharman:Seattle Genetics: Research Funding; Gilead: Research Funding; Celgene: Research Funding; Pharmacyclics: Research Funding; Acerta: Research Funding; TG Therapeutics: Research Funding. Flowers:Gilead: Consultancy, Research Funding; Millennium: Consultancy, Research Funding; Spectrum, Janssen, Infinity, AbbVie, Acerta, Pharmacyclics, TG Therapeutics: Research Funding; Optum Rx, Seattle Genetics, Genentech/Roche: Consultancy; Celgene Corporation: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal