Abstract
Background: The ASCO Value Framework was developed to assist in value assessments (efficacy, toxicity, and cost of therapies) of drugs in cancer care. However, data on its feasibility and clinical application have not been widely reported. Given the introduction of novel therapeutics for chronic lymphocytic leukemia (CLL), we aimed at assessing the feasibility and applicability of the Framework in this disease.
Objective: We used the ASCO Value Framework to evaluate and compare net health benefit and cost within Medicare of all regimens listed in the current National Comprehensive Cancer Network (NCCN) guidelines for CLL.
Methods: The current NCCN guidelines (Version 3.2016) for CLL were reviewed. All regimens for first-line and refractory/relapsed therapy were evaluated by literature review of the references listed. The trials were screened for appropriate use for the ASCO Value Framework, which is limited to randomized-controlled prospective trials. Additional randomized prospective trials published as of July 2016 were used if the arms contained regimens listed in the guidelines. Retrospective, phase I, single arm trials, trials comparing different dosing of the same regimen, and trials containing a control arm not listed as a treatment option per guidelines were excluded. The revised ASCO Value Framework (Schnipper et al., JCO 2016) was used to calculate net health benefit (derived from efficacy and toxicity of the regimens) using the advanced disease model. For the toxicity score (as described in the Framework), neutropenia, thrombocytopenia, and anemia were included as they are clinically relevant in CLL, however laboratory values were excluded. To compare drug acquisition cost, average sales price (ASP) from the July 1, 2016 Medicare Part B data was used to calculate 6 cycles or a complete course of infusional therapy based on an average 81.5 kg person with a body surface area of 1.96 m2 (dosing according to referenced studies). Retail cost and estimated cost-sharing (with drug plan coverage) of oral drugs were calculated using the medicare.gov plan finder based on a 6 month supply.
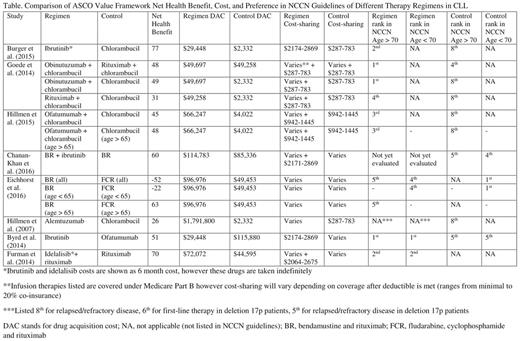
Results: Thirty-nine studies were included for screening, including all referenced studies in the NCCN guidelines and one additional trial. The following observations were made: 1. A total of eight studies (20%) could be evaluated by the ASCO Value Framework which included 13 possible comparisons between 12 different regimens (see Table). 2. It is able to demonstrate clinical benefit in contrast to cost in some situations. When comparing ibrutinib plus bendamustine and rituximab (BR) to BR alone, the framework is able to demonstrate a high clinical benefit of ibrutinib along with its high cost-sharing. It is also able to demonstrate that alemtuzumab has lower clinical benefit compared to other regimens studied against chlorambucil with a higher drug acquisition cost. 3. Toxicity scores are variable. Alemtuzumab demonstrates a high toxicity score which is expected. When comparing BR versus fludarabine, cyclophosphamide and rituximab (FCR), there was no major difference using the toxicity scores despite established clinical differences in toxicities. 4. Different net health benefit scores are calculated depending on the clinical variable (progression free survival, overall response rate) available (demonstrated with BR versus FCR). 5. When comparing ibrutinib, obinutuzumab plus chlorambucil, rituximab plus chlorambucil, and alemtuzumab to chlorambucil as a control the calculated net health benefit ranked these regimens similar to their preferred order in NCCN guidelines. 6. Six of the eight studies used less potent control arms (chlorambucil, rituximab alone) which are cheaper but are among the lowest preferred NCCN recommendations. 7. A direct comparison of ibrutinib versus chemoimmunotherapy is currently lacking and needed in clinical practice given its large cost difference (though trials are ongoing).
Conclusions: The ASCO Value Framework is a promising tool that helps compare cost and net health benefits. Major limitations when applied to CLL include limited number of trials that could be evaluated by the Framework, and lack of trials comparing stronger control arms. To optimize application of this Framework, we would urge investigators and sponsors to consider the assessment and reporting of the required variables to determine the net health benefit of therapies in clinical trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal