Abstract
Background: Patient (Pt) outcomes have improved significantly in multiple myeloma (MM) due to widespread use of novel therapeutic agents and stem cell transplant (SCT) butoutcome disparities among racial/ethnic groups exist. Differences in access to and utilization of novel therapeutics are likely contributing factors. We performed an analysis of the SEER-Medicare database to explore such differences.
Methods: Pts with a confirmed MM diagnosis between 2007-2009 and continuous Medicare coverage from 1 year prior to diagnosis through end of 2012 were included. Medicare Part D data (oral medications) is not available prior to 2007 so utilization analysis of lenalidomide (LEN), thalidomide (THAL) and bortezomib (BORT) was performed from 2007-2009. Demographic/survival data (PEDSAF files) and drug/SCT utilization (NCH, OUTSAF, PDESAF files) were obtained (HCPCS/NCH codes). Categorical and continuous variables were compared using /Fisher tests and Wilcoxon tests, respectively. Gender, age, and year-adjusted p-values were calculated using logistic regression. A proportional odds model was used to detect time-dependent trends in drug usage within 12 months of MM diagnosis across year of diagnosis by race. Separate multivariate models were fit for each drug adjusting for gender, age, drug use and race. Associations between drug use and overall survival (OS) were examined using a time dependent proportional hazards model.
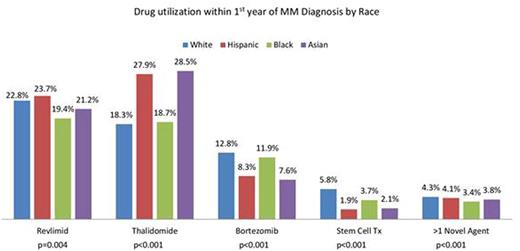
Results: A total of 5358 MM pts were included (any Medicare claims) with 2733 (51%) males. Mutually exclusive race categories were White (W; 67%), African-American (AA; 18%), Hispanic (H; 10%) and Asian (A; 5%). Median follow up was 2.4 yrs. Baseline pt characteristics were significantly different for age with AA being the youngest at diagnosis (p<0.001) and for gender (p<0.001) with W having the largest (53.4%) and AA the smallest (45.2%) proportion of males. Medicare claims capture only pts without any supplemental insurance and so drug utilization in this database was much lower than expected for all subgroups. Year-wise comparison showed that LEN use within the first year of MM diagnosis increased from 2007 to 2009 (16.5% to 27.7%; p<0.001) and there was a significant decrease in median days to first dose (p=0.002). Over the same period, use of THAL decreased (26.6% to 14.6%; p<0.001) while use of BORT (7.8% to 15.4%; p<0.001), SCT (3.3% to 6.2%; p<0.001) and more than 1 novel agent (2.5% to 5.7%; p<0.001) increased significantly. Utilization patterns within first year of MM diagnosis were significantly different among racial subgroups for LEN, THAL, BORT, SCT and >1 novel agent use. (Figure 1) No significant difference was seen for days to first dose of LEN/THAL for any race but for BORT, H got first dose in median 117 days after diagnosis as compared to median 46-51 days for other races (p=0.025). During the first year after diagnosis, W and AA had higher BORT only usage while H and A had a higher LEN/THAL only usage (p<0.001). For drug use by year and race, a significant increase in LEN use was seen from 2007 to 2009 in W (p<0.001), AA (p=0.001) and A (p<0.001) but not H (p=0.26) while THAL use decreased significantly for all races. BORT use increased significantly for all except A (p=0.602) and SCT use increased significantly for all except AA (p=0.068). Inferior OS was significantly associated with older age (p<0.001) and males (p<0.001). LEN/THAL use within the first year of diagnosis did not affect OS while BORT use within the first year of diagnosis increased mortality risk (HR 1.18, p=0.021). SCT use improved OS (HR 0.52, p<0.001).
Conclusions: In this comprehensive analysis of MM therapeutics utilization, we noted significant variability among different racial groups. The use of LEN, BORT and SCT has increased while THAL has decreased over time but disparities exist in utilization among different races, with lower use of LEN in AA, lower use of BORT in A and significantly delayed use of BORT in H, which are all notable since racial/ethnic minorities are fast growing in the US population mix and seem to have inequitable utilization and/or access. Inferior survival with early BORT use may represent selection bias of pts with more advanced/aggressive disease. These trends need to be examined in larger sample sizes especially from commercial payers to eliminate drug access and utilization disparities and achieve equitable benefit of therapeutic advances across all racial subgroups.
Ailawadhi:Pharmacyclics: Consultancy; Novartis: Consultancy; Amgen Inc: Consultancy; Takeda Oncology: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

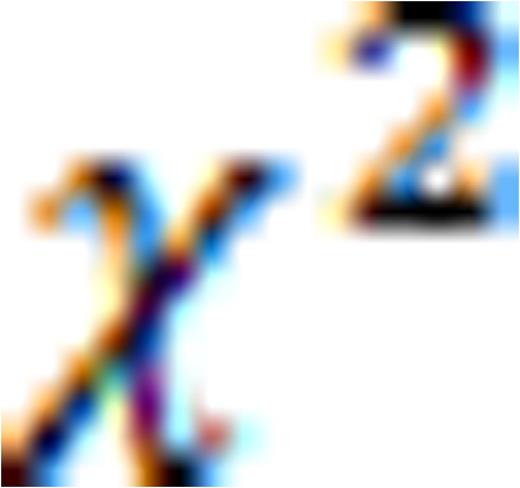
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal