Abstract
Introduction
Haploidentical allogenic hematopoietic stem cell transplants (haplo-HSCT) is an emerging alternative transplant procedure for patients with hematologic malignancies that are in need of transplant and do not have a compatible donor. Due to the broad HLA disparity, the haplo-HSCT can be performed with T-cell depletion and megadose of CD34+ cells. Alternatively haplo-HSCT can be performed with non T-cell depleted transplants (T-replete) either in combination with anti-thymoglobuline serum (ATG) or post-transplant cyclophosphamide (PT-Cy) as graft-versus-host disease (GVHD) prophylaxis strategy. No randomized study compared yet these strategies for GVHD prophylaxis in the setting of haplo-HSCT. Nevertheless, many centers tend to specialize in one GVHD prophylaxis strategy making it difficult to differentiate the treatment effect from the center effect. Center effect is a known risk factor for outcomes of haplo-HSCT in both T-cell depleted (TCD) and T-replete settings. The objective of the study was to investigate the role of center effects in GVHD prevention strategy, on leukemia-free survival (LFS) and overall survival (OS) in a population of adult patients with acute leukemia receiving haplo-HSCT.
Patients and methods
A retrospective multicenter registry study was conducted on patients reported to the European society for Blood and Marrow Transplantation registry. Inclusion criteria were: age > 18 years, lymphoblastic or myeloid acute leukemia (ALL or AML) in first or second complete remission (CR1 or CR2), receiving a haplo-HSCT between 2005 and 2014. In this population (n=606), in order to assess the interaction between center and GVHD prevention treatment, we then included in the study selected patients from the centers that had performed more than 20% of both TCD and T-replete haplo-HSCT during the study period. Center effects on the outcomes consisted of 1) center effect on the baseline risk of event and 2) interaction between center and strategy of GVHD prevention. These center effects were estimated respectively using Cox mixed-effects models and tested using permutation tests. All models were adjusted on patient age, CMV statuses, disease (ALL or AML), secondary leukemia, previous autologous transplant, disease status (CR1 or CR2), peripheral blood vs. bone marrow transplant and conditioning regimen.
Results
After selection, 226 patients were available across 29 centers in Europe. Most centers included less than 10 patients. One hundred and one (45%) patients received TCD, 125 T-replete haplo-HSCT (62 (27%) using ATG and 63 (28%) using PT-Cy). Median age at transplant was 50 years in TCD haplo-HSCT patients and 41 years in T-replete patients. Overall, 175 (77%) patients had AML. There were 86 (69%) peripheral blood transplants in the TCD group and 92 (91%) in T-replete. Median follow-up was 2.7 years and median survival was 1.8 years (95%CI: 1.2-4.2).
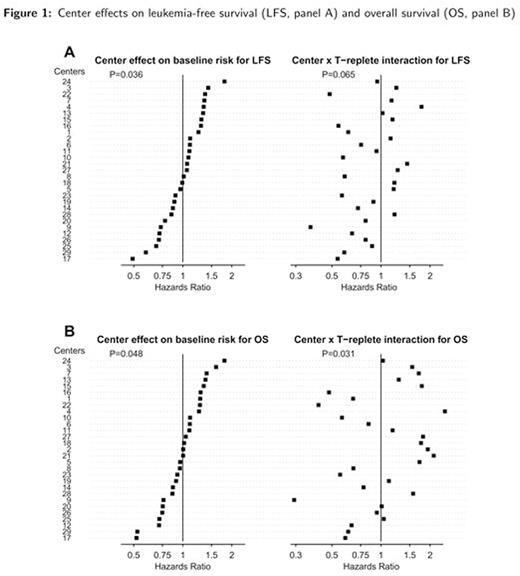
In adjusted analyses, without accounting for center effect, T-replete tended to be associated with better LFS (Hazard Ratio (HR): 0.70 (95%CI 0.45-1.07), p=0.10) and 0S (HR=0.67 (95%CI 0.43-1.04), p=0.076). When center effects were included, there was significant heterogeneity across centers on the baseline risk of both outcomes (LFS: p=0.036 and OS: p=0.048). When accounting for interaction between center and strategy for GVHD prevention, the effect of T-replete vs. TCD on the outcomes did vary across centers (p=0.065 and p=0.031 for interactions in LFS and OS, respectively). These were qualitative interactions: HR for OS associated with T-replete, compared to TCD, varied from 0.46 (better OS) to 1.63 (worse OS) when considering first and third quartiles of the center effects distribution (Figure 1).
Conclusions
We found an interaction between center and strategy for GVHD prevention on outcomes of patients who received a haplo-HSCT. The difference between the 2 strategies (TCD or T-replete) varied across centers and could even be reversed according to the center. This could be in part related to the increase in expertise with each technique in some centers and with the different management of complications, such as infections-related and relapse.
Ciceri:MolMed SpA: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal