Abstract
Background. Hypomethylating agents (HMA) are standard therapy for patients with higher-risk MDS and AML patients (pts) declining induction chemotherapy. However, >60% do not respond and all responding patients ultimately relapse. Prognosis after HMA failure is poor, with median overall survival (OS) of 4-6 months (m) and 2-year survival of 15%. Second-line intensive chemotherapy (IC) can reduce disease burden and serve as a bridge to allogeneic stem cell transplantation. To date, no large-scale study has focused on HMA failure pts and there is no standardized induction regimen. This retrospective, multicenter cohort study compared clinical responses, cumulative incidence of relapse (CIR), and median OS for different induction chemotherapy regimens given after HMA failure in MDS and AML pts.
Methods. Pts from 11 centers treated between January 2005 to September 2015 were analyzed. Inclusion criteria was age 18 years or older, diagnosis of higher-risk MDS (IPSS Int-2 or high) or AML, failure to respond or progression of disease after at least 1 HMA cycle, and treatment with an intensive chemotherapy (IC) regimen after HMA failure. Response was evaluated per AML 2003 IWG criteria (CR + CRi), OS was calculated from time of induction to death or last follow-up, CIR was calculated from time of response to IC to relapse or last follow-up. All statistical analyses were adjusted on region (Europe vs US).
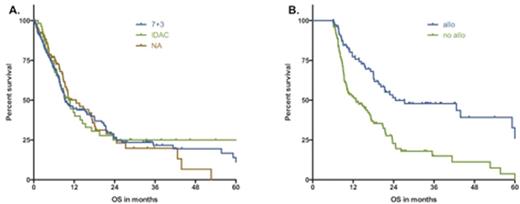
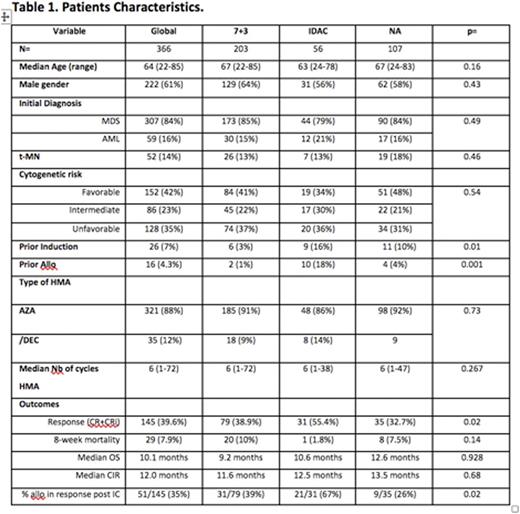
Results. Of 366 included pts, 203 received 7+3, 56 received intermediate to high-dose Aracytine (IDAC), and 107 received a nucleoside analogue (NA)-based regimen (fludarabine, cladribine, clofarabine). Baseline demographics (sex, age, IPSS cytogenetic risk stratification, and prior number of HMA cycles) were similar between the treatment groups (table1). For the whole cohort, the overall response rate to chemo was 39.6%, 8-week mortality was 7.9%, the median OS was 10m (95%CI [8-12m]) and the cumulative incidence of relapse was 50% at 1 year and 71% at 2 years. In univariate analyses, history of therapy related myeloid neoplasm, adverse cytogenetic risk, and use of 7+3 or NA vs IDAC negatively impacted chance of response. Cytogenetic risk stratification was the only variable that impacted response in multivariate analyses (MVA) (45%, 46%, and 28% for favorable, intermediate, and unfavorable group respectively, p=0.005). Factors associated with significantly shorter median OS in MVA included diagnosis of AML at the time of initiation of HMA (6m vs 11m for AML vs MDS, p=0.006), adverse cytogenetics (15m, 10m, 7m for favorable, intermediate, and unfavorable groups, respectively, p=0.003), prior IC before HMA (8m with vs 11m without IC, p=0.001), and progression of disease at the time of HMA failure (9m with vs 20m without, p<0.001). There was no impact on OS of the induction regimen (Figure 1A). The cumulative incidence of relapse after IC was significantly increased for patients receiving IC before HMA (median CIR of 8m with vs 14m without, p=0.006) and progression at the time of HMA failure (9m with vs 17m without, p=0.05). Allogeneic transplantation was performed in 95 pts (26%), including 53 pts in complete response after induction (37% of responders). In a landmark analysis performed at 6 months after IC, transplanted pts had improved OS vs non-transplanted pts (25m vs 13m, p<0.001, Figure 1B). Moreover, no long-term survival was observed in the cohort of responding patients who were not transplanted.
Conclusions. This large cohort of patients treated with IC after HMA failure confirms the relatively dismal outcome of this patient group. No induction strategy is superior for outcomes of interest, nor more toxic. IC-post-HMA is a valid treatment option, with response rates and transplant rates that exceed what is observed with other treatment modalities. The benefit of IC-post-HMA may be maximized by patient selection: non-adverse cytogenetics, no prior exposure to IC, and availability of an allogeneic donor seem to be the key factors.
A. Kaplan-Meier estimate of overall survival as of IC-post-HMA: impact of induction regimens B. 6-months overall survival landmark analysis: impact of allogeneic transplantation.
A. Kaplan-Meier estimate of overall survival as of IC-post-HMA: impact of induction regimens B. 6-months overall survival landmark analysis: impact of allogeneic transplantation.
Komrokji:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Speakers Bureau. Ades:Celgene, Takeda, Novartis, Astex: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Sekeres:Millenium/Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Pleyer:Celgene: Consultancy, Honoraria; Bristol-Myers-Squibb: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; AOP Orphan Pharmaceuticals: Honoraria. Vey:Sunesis: Honoraria. Almeida:Alexion: Speakers Bureau; BMS: Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau; Shire: Speakers Bureau. Gore:Celgene: Research Funding. Prebet:celgene: Consultancy, Honoraria; Novartis: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal