Abstract
Introduction:
The optimal management of multiple myeloma (MM) patients (pts) with t(4;14) is not certain. Before the introduction of novel agents, the progression-free (PFS) and overall survival (OS) outcomes with autologous stem cell transplant (ASCT) were disappointing (median PFS 9.9 months [mos]; Chang H, et al. Bone Marrow Transplant 2005;36:793). Subsequently, early experience with bortezomib (btz) in relapsed myeloma showed its efficacy in t(4;14) disease, and led to the prospective Canadian trial of 12 cycles of btz-based therapy (DBD = Doxorubicin + btz + dexamethasone) x 4, followed by CyBor-P (Cyclophosphamide + btz + prednisone) x 8 and then dexamethasone maintenance, without planned ASCT, in newly diagnosed pts with this cytogenetic abnormality by FISH (Reece D, et al; Blood 2010;116: abstract 1968). After completion of this trial, particularly with the absence of provincial funding for long-term upfront btz, we adopted a standard approach of btz-based induction followed by ASCT. We now report the results of newly diagnosed t(4;14) pts treated at our centre with these 2 approaches.
Methods:
We analysed consecutive patients with newly diagnosed MM harbouring t(4;14), who were transplant-eligible and treated at Princess Margaret Cancer Centre with btz-based therapy between July 1, 2006 and June 30, 2014. Patients lost to follow-up (F/U) before completion of front-line treatment or transferred to another centre after first-line treatment were excluded. Statistical analyses were performed using IBM SPSS version 20.
Results:
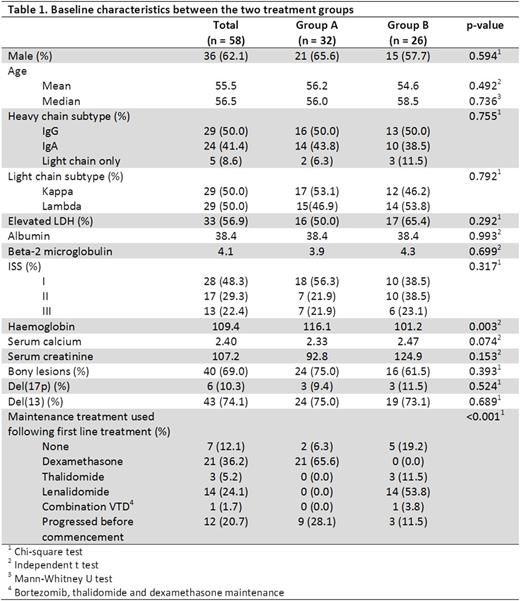
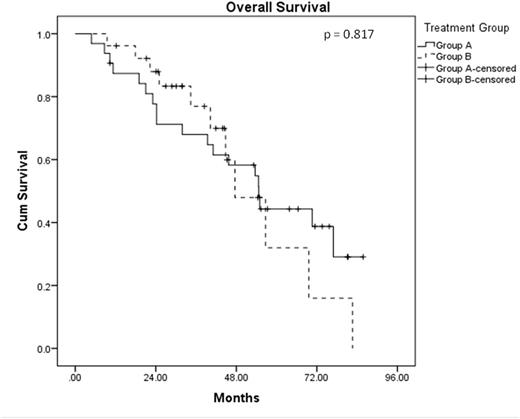
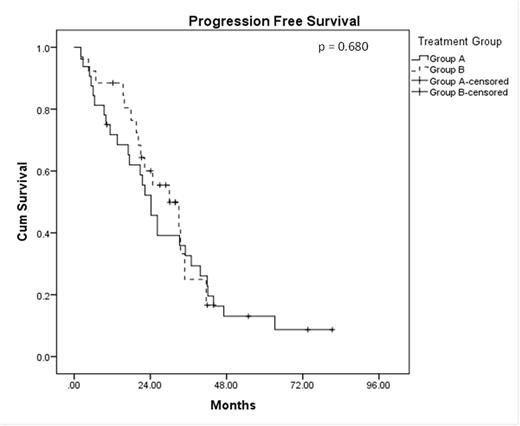
A total of 58 patients were analysed with a median F/U of 41.5 mos. The median age at diagnosis was 56.5 years, and 62.1% were male. Concomitant del(17p) was noted in 10.3% of the patients. Thirty-two of these pts (Group A) were enrolled onto the prospective phase II study described above; the other 26 pts (Group B) received the subsequent standard of care, which involved btz-based induction (usually CyBor-D) for 4-6 months, followed by planned ASCT. Amongst these 26 patients, 11 received single ASCT, 13 received tandem ASCT and 2 failed to respond to btz-based induction. The only significant difference in pt characteristics between these 2 groups was a higher median haemoglobin level in group A (116 g/L vs 106 g/L; p=0.003). The median OS was 54.7 months for Group A and 47.6 months in Group B (HR = 1.095, p = 0.817). Median PFS for Group A was 24.2 months and was 30.0 months for Group B (HR = 0.874, p = 0.680). No difference in OS and PFS was noted between those who received single and tandem ASCT as planned front-line treatment (OS HR = 1.105, p = 0.895; PFS HR = 1.723, p = 0.317). Amongst those in Group A, 25 patients have relapsed to date. Eight received single ASCT, 6 received tandem ASCT, and 11 received chemotherapy only as salvage therapy. In Group B, 15 patients have relapsed, and 11 of these went on to receive lenalidomide-based salvage treatment. The PFS and OS from the time of first relapse in this group of relapsed patients in Group A was similar between those who received ASCT as front-line treatment in Group B (median PFS 12.5 vs 11.1 months, p = 0.842; median OS 27.5 vs 21.6 months, p = 0.985). Amongst those who relapsed in Group A, those who received salvage ASCT had a better PFS and OS from the time of relapse than those who did not received salvage ASCT (median PFS 22.4 vs 1.7 months, p < 0.001; median OS 35.8 vs 11.7 months, p = 0.021). In multivariate analysis, only del(17p) was found to be an independent adverse risk factor for OS (HR = 5.307, p = 0.032). Age (HR = 1.050, p = 0.089) and ISS stage III (HR = 4.036, p = 0.097) were also noted to be adverse risks factor for OS.
Conclusions:
Newly diagnosed MM pts with t(4;14) treated with btz-based therapy with or without ASCT as part of first-line therapy have a median OS in the range of 4-4.5 years; 2) the use of ASCT at some time point in the course of t(4;14) disease is likely beneficial; 3) however, there was no obvious benefit for tandem ASCT as part of initial or salvage therapy in this analysis, although we cannot exclude an effect of pt bias in the ability to perform 1 or 2 ASCT; 4) the integration of an IMiD, particularly lenalidomide, in more pts either in combination with btz or as maintenance therapy, may have improved the outcome but was not available for all pts in this analysis; 5) as observed in other centres, the co-existence of del 17p and t(4;14) identifies a particularly high risk group of MM pts.
Chen:Takeda: Research Funding; Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Kukreti:Amgen: Honoraria; Celgene: Honoraria; Lundbeck: Honoraria. Prica:Janssen: Honoraria; Celgene: Honoraria. Tiedemann:Celgene: Honoraria; Janssen: Honoraria; BMS Canada: Honoraria; Novartis: Honoraria; Amgen: Honoraria; Takeda Oncology: Honoraria. Trudel:Celgene: Honoraria; Glaxo Smith Kline: Honoraria, Research Funding; Oncoethix: Research Funding; Novartis: Honoraria. Reece:Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Merck: Research Funding; BMS: Honoraria, Research Funding; Otsuka: Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal