Abstract
Background: Among patients with immunoglobulin light chain (AL) amyloidosis, there is little consensus on when reinstitution of chemotherapy should occur (Milani and Dispenzieri, International Society of Amyloidosis 2016). AL amyloidosis patients who are treated with high dose chemotherapy followed by autologous stem cell transplant (ASCT) are a relatively low-risk and homogenous population, making them an ideal group to study practice patterns.
Methods: We conducted a retrospective study to evaluate the patterns of relapse or progression and the timing of re-initiation of therapy among 146 patients who were initially treated with ASCT at Mayo Clinic between 1996 and 2009 and who received second-line therapy between 7/9/1997 and 4/12/2012.
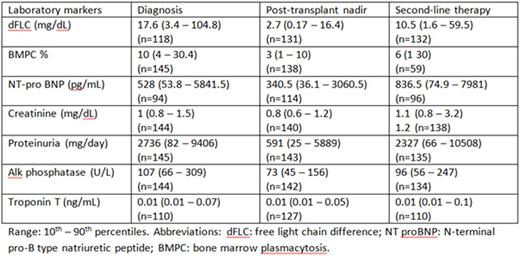
Results: The median time from ASCT to second-line therapy was 23.6 months and the median follow up post ASCT was 57.5 months. The indications for second-line treatment were: 1) both hematologic and organ progression 24.7% (36 patients); 2) organ progression only 41.1% (60); 3) hematologic relapse only 34.2% (50). The median dFLC at the time of starting second-line therapy was 10.5 mg/dL (1.6 - 59.5 mg/dL), which was 44.9% (13.8-167.2%) of dFLC level at diagnosis. Increase in proteinuria by > 50% from nadir (that was also at least 1g/24 hours, i.e. renal progression) was present in 35.8%. Increase in NT proBNP by >30% from nadir and minimum of 300 pg/mL was present in 48.9% of patients. The respective 4 years overall survival rates from the time of ASCT were 87.8%, 63.9%, and 56.7% (p=0.0016) for patients who had hematologic relapse, organ progression only and both organ and hematologic progression. Comparisons of laboratory markers at diagnosis, nadir of post ASCT and initiation of second-line therapy are listed in the table.
Conclusions: Our study investigated the patterns of relapse / progression following upfront ASCT. This provides some insights on practice patterns of when physicians re-initiate therapy.
Gertz:NCI Frederick: Honoraria; Celgene: Honoraria; Med Learning Group: Honoraria, Speakers Bureau; Research to Practice: Honoraria, Speakers Bureau; Novartis: Research Funding; Prothena Therapeutics: Research Funding; GSK: Honoraria; Sandoz Inc: Honoraria; Ionis: Research Funding; Alnylam Pharmaceuticals: Research Funding; Annexon Biosciences: Research Funding. Kumar:Millennium: Consultancy, Research Funding; Array BioPharma: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Noxxon Pharma: Consultancy, Research Funding; AbbVie: Research Funding; Glycomimetics: Consultancy; Janssen: Consultancy, Research Funding; BMS: Consultancy; Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Onyx: Consultancy, Research Funding; Kesios: Consultancy. Kapoor:Celgene: Research Funding; Amgen: Research Funding; Takeda: Research Funding. Dispenzieri:Prothena: Membership on an entity's Board of Directors or advisory committees; Alnylam: Research Funding; Celgene: Research Funding; Jannsen: Research Funding; pfizer: Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal