Abstract
Introduction: Acute graft-versus-host disease (aGVHD) is a leading cause of transplant related mortality following allogeneic hematopoietic stem cell transplantation (HCT). The most widely used aGVHD prophylaxis regimen is a combination of a calcineurin inhibitor and a short course of methotrexate. Results of studies on the optimal blood levels of the calcineurin inhibitor tacrolimus to prevent aGVHD are mixed, with some showing no correlation and others indicating that trough levels between 7-15 ng/mL required to adequately prevent aGVHD. Currently, the established target trough level for this agent is 10 to 20 ng/mL. We retrospectively evaluated the relationship between tacrolimus blood concentration and the incidence of grade II-IV aGVHD in a large cohort of adult patients following allogeneic HCT from either an HLA-matched related or matched unrelated donors.
Methods: We evaluated all related/unrelated allografts between January 2004 and December 2012 at the Cardinal Bernardin Cancer Center at Loyola University. Inclusion criteria included age 18 years or older, first allogeneic HCT for a hematological malignancy, and use of tacrolimus and short term methotrexate for aGVHD prophylaxis. Donors included human leukocyte antigen (HLA) identical sibling (Allo-Sib) or unrelated donors (MUD) either fully matched or 1 antigen mismatched (10%). Both myeloablative and non-myeloablative conditioning regimens were included. Disease risk was defined by the ASBMT classification and Co-Morbidity Index by Sorror et al (HCT-CI). Infusional tacrolimus was initiated on day -2 of HCT at dose of 0.03 mg/kg/d and AM trough blood concentrations measured every other day during the entire hospital stay with the goal of maintaining a level of 10-15 ng/ml and then twice weekly after discharge. Dose adjustments or withholds were based primary on serum creatinine and trough levels per a set protocol that withholds for levels greater than 20 ng/ml or creatinine greater than 2.0 mg/dl. Acute GVHD was graded based on the International Bone Marrow Transplant Registry System. Univariate and multivariate analyses exploring all risk factors for aGVHD were performed.
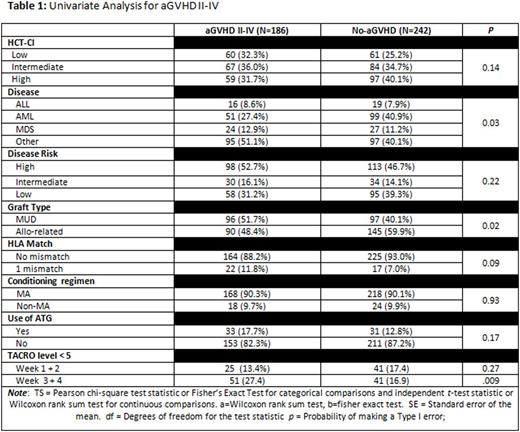
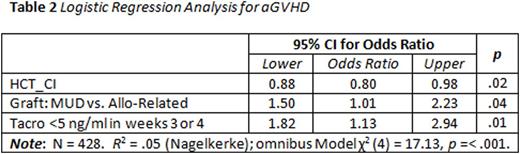
Results: A total of 428 patients met the inclusion criteria. Median age was 48.5 years with a male predominance (60.6%). HCT-CI was evenly distributed with 121 (28.2%) low risk, 151 (35.3%) intermediate risk, and 156 (36.4%) high risk. Per ASBMT disease risk classification, more patients had high disease risk (n=211, 49.3%); 64 (15%) with intermediate risk, and 153 (35.7%) with low risk. Acute GVHD II-IV developed in 186 (43%) patients in this largely myeloablative patient population (89%), with donor source split nearly evenly between MUD (45%) and Allo-sib (55%). An extensive univariate analysis for the factors associated with the development of aGVHD was developed (Table 1). The strongest predictor of aGVHD was a level of tacrolimus < 5 ng/ml in weeks 3 and 4 after transplant: of those with no aGVHD only 16.9% had a level < 5 ng/ml during these weeks vs 27.4% with aGVHD (p = .009). Other tacrolimus trough cut points, i.e. 10 ng/ml, 2.5 ng/ml and the mean (13.4 ng/ml in each group) were not associated with a higher rate of aGVHD. In the univariate analysis, AML and allo-sib grafts were noted to be associated with lower risk of aGVHD. In multivariate analysis (Table 2), tacrolimus levels less than 5 ng/ml in weeks 3 or 4 was found to be the most statistically significant factor in predicting aGVHD incidence (Odds ratio 82 95% CI 1.13-2.94; p = .01); other significant factors include HCT-CI (p = .02) and graft source (p = .04).
Conclusion: In one of the largest retrospective studies examining the correlation of early tacrolimus blood concentrations and acute GHVD incidence, we show that trough tacrolimus levels less than 5 ng/ml in weeks 3-4 following Allo-Sib or MUD HCT is a strong predictor for an increased risk of aGVHD. We affirm that higher HCT-CI and donor source are risk factors for aGVHD after allografts. This not only helps define more accurately the lower limits of acceptable tacrolimus levels, but appears to emphasize the importance for close monitoring and dose adjustment of these levels at a time when patients are typically discharged to the outpatient setting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal