Abstract
Background: CD101 is a novel echinocandin with long-acting pharmacokinetics and exceptional stability in development for prevention and treatment of serious fungal infections. The in vivo efficacy of CD101 was evaluated using neutropenic mouse models of azole-resistant candidiasis and aspergillosis.
Methods: An azole-resistant strain of Candida albicans (R357; resistant to fluconazole [Flu], voriconazole, and posaconazole but susceptible to amphotericin B [AmB] and echinocandins) isolated from human blood was used for the mouse candidiasis model. A test strain of Aspergillus fumigatus (ATCC 13073) was used for the mouse aspergillosis model. Mice were rendered neutropenic by cyclophosphamide and then infected by injections of C. albicans (105 CFU/mouse) or A. fumigatus (104 CFU/mouse) into the tail vein. Test articles were administered starting 2 hours after infection. In the mouse candidiasis model, groups of 5 mice each received one dose of AmB (3 mg/kg IV), Flu (20 mg/kg orally), or CD101 (3, 10 or 30 mg/kg by intraperitoneal administration [IP]). After 72 hours post-infection, mice were euthanized and C. albicans counts in kidney tissue (CFU/g) were measured. In the mouse aspergillosis model, groups of 10 mice each received one dose ofAmB (2 mg/kg IP) or CD101 (2 mg/kg IV and IP). Survival was monitored daily for 10 days. Differences between vehicle and test article groups were assessed for significance by one-way ANOVA followed byDunnett's test and Fisher's Exact test in the candidiasis and aspergillosis models, respectively.
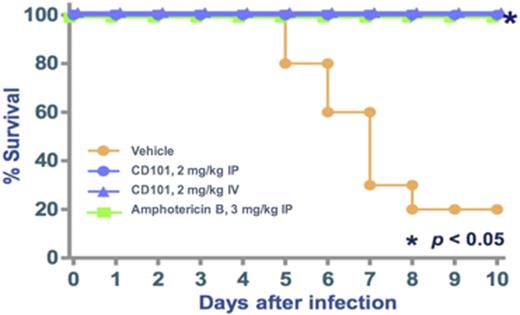
Results: One dose of CD101 3 mg/kg produced a >99.9% (or > 3-log; P<0.001) reduction in C. albicans CFU compared with vehicle through at least 72hours post-dose following a single IP dose.AmB showed similar, albeit less robust, efficacy (>99% or >2-log reduction in CFU; P<0.05), whereas fluconazole was less efficacious (83.9% or <2-log reduction in CFU). In the aspergillosis model, CD101 administered 2 mg/kg IV or IP showed similar efficacy to that ofAmB 2 mg/kg IP, both with significantly longer survival than vehicle (P<0.05; Figure).
Conclusions: A single dose of CD101 3 mg/kg produced significant reduction in C. albicans burden compared with vehicle (P<0.001) in the neutropenic mouse model of azole-resistant candidiasis, demonstrating efficacy comparable, if not better, to that of AmB at the same dose. One dose of CD101 also demonstrated efficacy in the mouse model of aspergillosis. These data support the continued development of CD101 for treatment of serious infections caused by Candida, including azole-resistant strains, and Aspergillus spp.
Ong:Cidara Therapeutics, Inc.: Employment. Bartizal:Cidara Therapeutics, Inc.: Employment, Other: Stockholder. Miesel:Eurofins Panlabs: Employment; Cidara Therapeutics, Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal