Abstract
Human T memory stem cells (TSCM) are a unique memory T cell subset with enhanced self-renewal capacity and the ability to differentiate into potent effectors could be valuable weapons in adoptive T-cell therapy against cancer. The distribution of TSCM cells in umbilical cord blood and leukemia/lymphoma remain unclear. In this study, we investigated the distribution of TSCM and other memory T (TM) subsets in the cord blood (CB) (n = 11), peripheral blood (PB) of lymphoblastic leukemia/lymphoma (LL) (n = 7), Myeloid leukemia (ML) (n = 7) as well as healthy individual (HI) (n = 16) by multicolor flow cytometry.
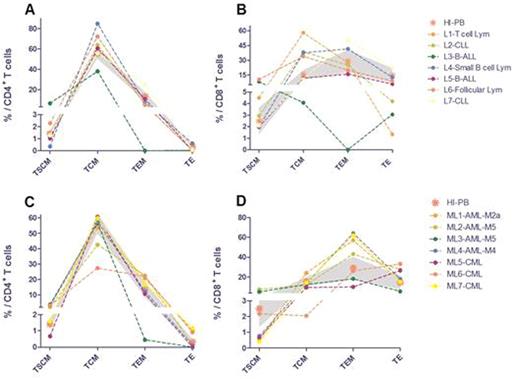
There were high percentage of CD4+ TSCM and CD8+ TSCM in CB group than that in HI-PB, however, fewer CD4+T central memory (TCM), CD4+T effector memory (TEM), CD8+ TEM and CD8+ terminal effector (TE) cells in CB group than in HI-PB (Figure 1). When compared LL or ML group with HI group, there was an increase in the CD4+ TSCM in the ML group, while the frequencies of TCM, TEM, or TE were similar. However, the percentage of memory T cell subsets in patient group was quite disperse, for example, CD4+ TSCM could range from 0.35% to 6.52% (LL) and from 0.67% to 3.71%(ML), the percentage of CD8+ TSCM could even reach to 10.43% and 7.62% in LL and ML group respectively, the same dispersion also exist in other T cell subsets of patient group but not in the blood and cord blood from HI. According to individually analyze distribution of T cell subsets in each patients, a higher percentage of CD4+ TSCM was detected in a B-ALL patient in the LL group comparing with HI, combined with significant lower percentage of CD4+ TCM, TEM and TE (Figure 2 A, L3), similar distribution pattern of CD8 T cell subsets also detected in the same B-ALL patient (Figure 2B, L3). Interesting, a case with BCR-ABL+ B-ALL include in this study showed a relative normal distribution pattern (Figure 2 A and B, L5). There were four patients in LL group showed a relative higher percentage of CD8+ TCM (Figure 2 B, L1, L2, L4 and L6), while two of them followed with quite lower TE in PB (Figure 2 B, L2 and 6). In the ML group, relative higher CD4+ TSCM combined with lower TEM was also detected in a case with AML-M5 (Figure 2 C, ML-3). In terms of CD8 T cell subsets distribution in ML group, we found two patients with AML-M5 possessing a higher CD8+ TSCM proportion compare with HI and other patients in ML group (Figure 2 D, ML-2 and 3).
In conclusion, we characterized the distribution of different memory T cell subsets in healthy CB and PB, the reason of high percentage of TSCM in CB is needed further investigation. In addition, the higher CD4+ TSCM detected in ML group and individual cases of LL group may related to the leukemia antigens they encountered, whether such a characteristic would impact their prognostic worth further investigation. Overall, the distribution of TSCM and other memory T cell subsets in blood between healthy and leukemia is not shown obviously difference, while, the large difference among individual patients reflect the various immunosuppression degree and immune reaction ability to tumor antigens. To characterize the relationship between distribution pattern of TSCM and TM cells and immune status of patients will benefit to developing more suitable individually immune treatment strategies.
Acknowledgments: The study was supported by grants from the National Natural Science Foundation of China (Nos. 81270604, U1301226, and 81400109), the China Postdoctoral Science Foundation (No. 2013M540685), Guangdong Natural Science Foundation (Nos. S2013040016151 and S2013020012863), the Foundation for High-level Talents in Higher Education of Guangdong, China (No. [2013]246-54), and the Guangzhou Science and Technology Project Foundation (201510010211).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal