Abstract
Background: EU PASS is an observational, noninterventional study designed to investigate the safety of lenalidomide (LEN) and other agents in the treatment of RRMM in a real-world setting.
Aims:To assess the incidence of adverse events (AEs) of special interest, including neutropenia, thrombocytopenia, venous thromboembolism (VTE), peripheral neuropathy (PN), and second primary malignancies (SPMs) in RRMM patients (pts) treated with LEN and other antimyeloma therapies according to current clinical practice.
Methods: Pts with RRMM who were commencing LEN treatment were enrolled at the investigator's discretion into a LEN cohort (LEN + dexamethasone, the approved combination for the treatment of RRMM); pts who received ≥ 1 prior therapy and were commencing a non-LEN-based therapy were enrolled into a background cohort (all other treatments, including novel agents). Thromboprophylaxis was per local standard practice. AEs were graded according to National Cancer Institute-Common Terminology Criteria for Adverse Events (version 3). SPMs were defined using Medical Dictionary for Regulatory Activities (MedDRA) terms under the category Neoplasms SOC. Following protocol amendment in 2011, assessments for SPMs were to be conducted up to 36 mos after treatment discontinuation.
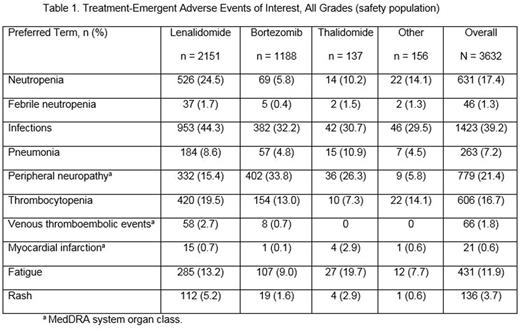
Results: As of June 2016, 3632 pts across 269 institutions in 17 European countries were included in the safety population. Of those, 59.2% received LEN (n = 2151), 32.7% received bortezomib (BORT; n = 1188), 3.8% received thalidomide (THAL; n = 137), and 4.3% received other therapies (n = 156). The majority of pts had discontinued from treatment (97.9%; n = 3556); of the 2.1% (n = 76) ongoing pts, 66 are treated with LEN, 6 with BORT, 0 with THAL, and 4 with other substances. Baseline characteristics were similar across the cohorts. Median age was 70 yrs (range, 25-95 yrs) and 54.0% were male. Of 2985 pts with available ECOG data, 2865 (96.0%) had good performance status (ECOG score 0-2), and the remaining 4.0% had an ECOG score of 3/4. The median number of prior therapies was 1 (range, 1-6) but was higher in the LEN cohort (2; range, 1-6) than in the BORT (1; range, 1-6) and THAL (1; range, 1-5) cohorts; the proportion of pts with only 1 prior treatment was also lower in the LEN cohort (44.3%), whereas BORT was 70.8% and THAL 56.2%. Overall, 50.7% of pts (n = 1842) had grade 3/4 AEs. Grade 3/4 neutropenia occurred in 17.1%, 3.5%, and 4.4% of pts in the LEN, BORT, and THAL cohorts, respectively, and grade 3/4 thrombocytopenia in 9.2%, 7.3%, and 3.6%. The incidence rate of SPM was 3.63 per 100 pt-yrs, with 3.18 per 100 pt-yrs in the LEN cohort, 5.23 per 100 pt-yrs in the BORT cohort, 2.73 per 100 pt-yrs in THAL, and 6.48 per 100 pt-yrs in others. AEs of interest of all grades are listed in Table 1. The median duration on study treatment was 6.6 mos (range, 0.1-81.6 mos) for LEN, 4.1 mos (range, 0-63.6 mos) for BORT, and 4.6 mos (range, 0.2-36.9 mos) for THAL. Treatment discontinuation rate due to AEs was similar in each cohort (22.1% in the LEN, 20.0% in the BORT, and 21.2% in the THAL cohorts). In the LEN cohort, dose reductions occurred in 38.1% of pts, with a median time to first dose reduction due to AEs of 12.4 weeks. Treatment-emergent adverse events leading to dose reductions were similar across cohorts, with 23.7% in the LEN cohort, 21.4% in the BORT cohort, and 17.5% in the THAL cohort.
Conclusions: Results of this noninterventional study in RRMM show that AEs were similar across cohorts except for higher rates of neutropenia and lower rates of PN with LEN compared with THAL or BORT. Higher rates of neutropenia did not translate into increased febrile neutropenia. Infections, independent from neutrophil counts, occurred in all cohorts, but few pts developed serious infections such as pneumonia. VTEs as well as myocardial infarctions were low throughout all cohorts. The occurrence of SPMs was generally low and comparable between cohorts. LEN was generally well tolerated.
Tholouli:Johnson and Johnson: Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Celgene: Honoraria; MSD: Speakers Bureau; Giles: Speakers Bureau. Hájek:Janssen: Honoraria; Takeda: Consultancy; BMS: Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Research Funding. Minnema:Celgene: Consultancy; BMS: Consultancy; Amgen: Consultancy; Jansen Cilag: Consultancy. Dimopoulos:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genesis: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Frost Andersen:Celgene: Research Funding. Waage:Amgen: Speakers Bureau; Celgene: Consultancy, Honoraria; Novartis, Amgen, Celgene: Membership on an entity's Board of Directors or advisory committees. Crotty:BMS, Takeda, Novartis, Janssen, Roche: Honoraria. Kueenburg:Celgene International Sarl: Consultancy, Honoraria. Di Micco:Celgene: Employment. Bacon:Celgene: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal