Abstract
Background
Phase 2 data from the RETRIEVE study suggested that retreatment with bortezomib IV ± dexamethasone in relapsed/refractory MM is active, adequately tolerated and feasible. The combination of bortezomib IV with dexamethasone in RETRIEVE, although associated with increased toxicity, was more efficacious than single-agent bortezomib (Petrucci T, et al. Haematol. 2010;95:152). SC administration of bortezomib has also shown improved tolerability, in terms of decreased side effects and drop-out rates, compared with the IV route (Moreau P, et al. Lancet Oncol. 2011;12:431-440). Here we report results of a randomized, international, multicenter, open-label phase 3 study that investigated the effects of experimental retreatment with SC bortezomib + dexamethasone followed by prolonged bortezomib therapy vs standard bortezomib + dexamethasone retreatment (NCT01910987; MMY3033). This study was terminated prematurely with insufficient sample size to adequately compare the retreatment therapies; the results should therefore be considered descriptive.
Methods
Pts were randomized (1:2) to receive either standard or experimental retreatment, stratified by the number of prior therapy lines. In the standard group, pts received 8 x 21-day cycles of SC bortezomib 1.3 mg/m2 (days 1, 4, 8, 11) plus dexamethasone 20 mg (days 1, 2, 4, 5, 8, 9, 11, 12). In the experimental group, pts followed this schedule for the first 2 cycles, followed by 4 x 35-day cycles of SC bortezomib (days 1, 8, 15, 22) plus dexamethasone (days 1, 2, 8, 9, 15, 16, 22, 23); pts completing experimental therapy were re-randomized (1:1) to prolonged single-agent SC bortezomib 1.3 mg/m2 (either on days 1, 8, 15, 22 of 35-day cycles or every other week) until disease progression, unacceptable toxicity, start of alternative MM therapy, withdrawal, or death. The primary endpoint was progression-free survival (PFS); secondary endpoints included overall response rate (ORR), duration of response (DOR), time to progression (TTP), overall survival (OS), time to next myeloma therapy (TTNT) and safety. Response and progression were assessed by investigators and the independent Data Monitoring Committee according to International Myeloma Working Group (IMWG) criteria. Descriptive analyses were performed in the intent-to-treat (ITT) population; time to event endpoints were analyzed using Kaplan-Meier methodology.
Results
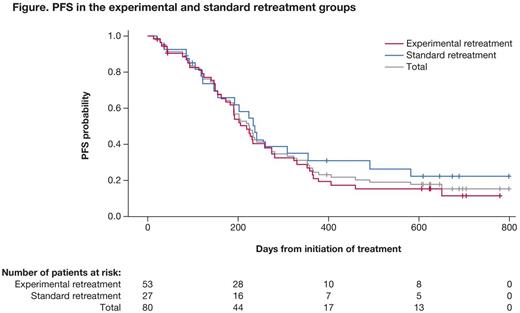
In total, 80 pts were randomized (experimental n=53, standard n=27), of whom 31 in the experimental group went on to receive prolonged bortezomib. In the experimental and standard groups, 53% vs 67% were male, median age was 68 vs 65 yrs, and 45/42/13% vs 48/48/4% had an ECOG PS of 0/1/2. After a median follow-up of 21.2 and 20.0 mos in the experimental and standard arms, respectively, and with 45 (85%) and 20 (74%) progression/death events, the median PFS was 7.2 mos (95% CI: 5.7-9.0) vs 7.8 mos (4.9-11.7) (Figure).
The ORR (complete + very good partial + partial responses) was 66% and 56% for experimental and standard retreatment, respectively. Median DOR was 6.5 vs 5.6 mos, median TTNT was 8.6 vs 9.0 mos, and median TTP was 7.4 vs 7.8 mos with experimental vs standard retreatment. Median OS at 18 months was 84% and 77%, following experimental and standard retreatment, respectively. All pts experienced at least one treatment-emergent adverse event (AE). The most common grade ≥3 AEs were (experimental vs standard; ≥5% in total population): thrombocytopenia (9% vs 22%), anemia (8% vs 7%), pneumonia (8% vs 4%) and atrial fibrillation (6% vs 7%); peripheral neuropathies (any grade; including peripheral neuropathy and peripheral sensory neuropathy) were reported in 23% vs 37% of pts. Serious AEs were reported in 34% vs 44% of pts in the experimental vs standard groups; the most common were atrial fibrillation (6% vs 4%), bronchitis (6% vs 0), pneumonia (8% vs 7%), and hypercalcemia (0 vs 7%). There were 6 on-study deaths due to treatment-emergent AEs (experimental: 5, standard: 1); all were considered unrelated to bortezomib or dexamethasone.
Conclusions
MMY3033 showed no significant efficacy benefit with experimental vs standard bortezomib retreatment therapy; however the study results were not sufficiently powered because of insufficient sample size. Efficacy and safety were consistent with previous reports of bortezomib/dexamethasone, with a slight reduction in thrombocytopenia events in the experimental retreatment group.
Cavo:Janssen-Cilag: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Millennium: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria. Gobbi:Janssen: Consultancy, Honoraria; Mundipharma: Consultancy, Research Funding; Takeda: Consultancy; Novartis: Consultancy, Research Funding; Gilead: Honoraria; Celgene: Consultancy; Roche: Honoraria. Potamianou:Janssen-Cilag Pharmaceuticals SACI: Employment. Lahaye:Janssen-Cilag: Employment. Couturier:Janssen-Cilag: Employment. Terpos:Celgene: Honoraria; Novartis: Honoraria; Genesis: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal