Abstract
Introduction: Hematologic response criteria for light chain amyloidosis (AL) requires that difference in involved and uninvolved free light chains (dFLC) be at least 5 mg/dL (or 50 mg/L). However, many patients do not meet these criteria and are often excluded from clinical trials. These patients are challenging to follow clinically as organ response takes much longer and therefore response to treatment is difficult to evaluate in the first few cycles. This study aims to evaluate patients who had non-evaluable FLC (dFLC< 5 mg/dL) at diagnosis and compare them to those who had evaluable FLC (dFLC≥ 5 mg/dL).
Methods: All patients with newly diagnosed AL seen within 90 days of diagnosis at our institution over a 10-year period (2006-2015) were identified from an institutional database. Data pertaining to demographics, diagnosis, treatment and follow-up was extracted from electronic medical records. Analysis was carried out by chi-square and Fisher's exact test for categorical variables and Kruskal-Wallis and Wilcoxon rank sum test for ordinal and continuous variables. Progression free survival (PFS) is defined as time to progression requiring treatment change or relapse requiring re-institution of treatment or death. PFS and overall survival (OS) were analyzed via the Kaplan-Meier method.
Results: Of 1336 patients meeting inclusion criteria, dFLC at diagnosis was known in 1290. 85.4% (n=1101) had dFLC ≥ 5 mg/dL, while 14.6% (n=189) had non-evaluable FLC. Median age at diagnosis (65.2 vs. 63.9 years), gender distribution (males 56.1% vs.64.8%) and involved FLC (lambda: 72.2% vs. 72.9%) was similar in FLC < 5 mg/dL and FLC ≥ 5 mg/dL group. Cardiac (38.1 vs. 76.3%, p <0.0001) and liver (10.2% vs. 16.3%, p=0.03) organ involvement were less common in patients with non-evaluable FLC (table 1). NT-ProBNP was significantly lower in the group with dFLC < 5 mg/dL in patients with and without cardiac involvement, as was Mayo cardiac stage (table 1). A trend towards less gastrointestinal (GI) involvement (17.1% vs. 24%, p=0.09) was also seen with dFLC < 5 mg/dL. In contrast, a trend towards higher renal involvement was seen in patients with dFLC < 5 mg/dL (64.6% vs. 55.9%, p=0.08), though this was not statistically significant. Median 24 hour urine protein was significantly higher in all patients (with and without renal involvement) with dFLC < 5 mg/dL compared to dFLC ≥ 5 mg/dL group (table 1).
Treatment details are listed in Table 1. ASCT (autologous stem cell transplant) was utilized more commonly in patients with dFLC < 5 mg/dL compared to patients with dFLC ≥ 5 mg/dL(43.2% vs. 26.1%, p <0.0001), including ASCT alone without chemotherapy (35.4% vs. 15.3%, p <0.0001).Rates of cardiac response (53.3% vs. 50.3%, p=0.88), and time to response (27.7 weeks vs. 35.6 weeks, p=0.67), were similar in both groups. Similarly, there was no difference in rates of renal and liver response and time taken to achieve a response (table 1). In patients with evaluable FLC, hematologic response was complete response (27.3%, n=245), very good partial response (21%, n=189), partial response (18%, n=160), no response (8%, n=74), progression (2%, n=15) and not known in 26.1% (n=216).
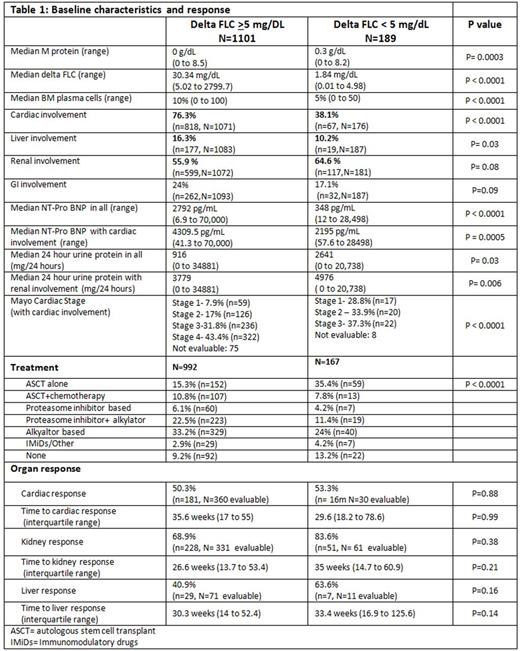
In patients who had follow up data available, 30.6% (44/144) with dFLC < 5mg/dL experienced a relapse/progression with median PFS of 4.1 years (95% confidence interval (CI): 3 to 5.7), while 34.7% (304/875) with FLC ≥ 5 mg/dL experienced a relapse/progression with median PFS of 1.3 years (95% CI 1.1 to 1.5); p<0.0001. Median OS was higher in patients with dFLC < 5 mg/dL at diagnosis at 8.3 years compared to 2.4 years in patients with dFLC ≥ 5 mg/dL (p < 0.0001) as depicted in Figure 1.
Conclusions: Patients with non-evaluable FLC at diagnosis have significant differences in organ involvement and survival compared to those with FLC ≥ 5 mg/dL at diagnosis. They have less cardiac and liver involvement and a trend towards less GI involvement, which may be secondary to low serum FLC burden and consequent less organ deposition. However, a trend towards higher renal involvement was seen in dFLC < 5 mg/dL group, with significantly higher urinary protein excretion. Loss of FLC in urine may result in lower serum FLC levels in this group. Survival was significantly better in patients with dFLC < 5 mg/dL, which may be explained by less cardiac involvement, lower cardiac stage and lower median FLC at diagnosis.
Dispenzieri:GSK: Membership on an entity's Board of Directors or advisory committees; Prothena: Membership on an entity's Board of Directors or advisory committees; pfizer: Research Funding; Celgene: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Alnylam: Research Funding; Jannsen: Research Funding. Kapoor:Amgen: Research Funding; Takeda: Research Funding; Celgene: Research Funding. Kumar:Celgene: Consultancy, Research Funding; Janssen: Research Funding; Sanofi: Consultancy, Research Funding; Skyline: Consultancy, Honoraria; BMS: Consultancy; AbbVie: Research Funding; Noxxon: Consultancy, Honoraria; Amgen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal